
The in vitro effect of fluorescein exposure on human corneal endothelial cells
Highlight box
Key findings
• Human corneal endothelial cell (HCEnC) health is not impacted by short-term (up 30 minutes) fluorescein at concentrations commonly used in clinical practice (0.01–0.2%). Furthermore, cells recovered from 60 minutes fluorescein within 24 hours. Continuous exposure to fluorescein concentrations as low as 0.001% over 24 hours induced significant and irreversible reductions in cell health.
What is known and what is new?
• Anterior segment fluorophotometry in humans and animal models of exposure keratopathy show that ocular surface fluorescein diffuses from the epithelium into the anterior chamber. Fluorescein effects on human corneal endothelium are not well studied.
• We provide new insights into how fluorescein can affect HCEnC health. The findings serve as a foundation for in vivo studies that could explore the effects of topical fluorescein for people with increased vulnerability to corneal damage.
What is the implication, and what should change now?
• Prolonged fluorescein exposure can impair HCEnC viability. Caution is needed when applying fluorescein in individuals with compromised corneal epithelial barriers, as seen for example with severe dry eye.
Introduction
Fluorescein sodium, more commonly referred to as ‘fluorescein’, is a synthetic organic fluorescent dye widely used in ophthalmology via different routes including topical, intravenous injection (e.g., fluorescein angiogram) (1,2), and less commonly oral and intracameral injection (e.g., for capsulorhexis during white cataract removal) (3). Fluorescein eye drops are used topically in concentrations commonly ranging from 0.5–2% for the evaluation of corneal abrasions and ulcers (3), contact lens wear fit (4), corneal surface structure, and to measure tear film break-up time most often in patients with dry eye disease (5,6). There is existing evidence on fluorescein toxicity in vivo; however, there have been limited studies on fluorescein cytotoxicity, especially the effects of fluorescein on corneal endothelial cells (1,2,7).
Topical application of fluorescein to the ocular surface results in it being distributed into the tears which then diffuses from the epithelium into the anterior chamber, as detected by anterior segment fluorophotometry in normal human subjects and in exposure keratopathy animal models (8,9). The small molecular weight of fluorescein (376 Da) allows it to pass through the porous structure of the corneal stroma. However, due to its relatively hydrophilic nature, fluorescein encounters resistance by the corneal epithelium and endothelium tight junctions (10). Transport across the endothelium can occur by two routes, the paracellular route where transport occurs through the tight junctions, and the transcellular route where transport occurs across the cells (11,12). In a study by Shiraya and Nagataki (13) the diffusion coefficient for fluorescein transport across the rabbit corneal stroma was found to be (0.94±0.11)×10−6 cm/sec with endothelial permeability of (4.7±1.0)×10−4 cm/min.
The human corneal endothelium is a 3–5 µm thick single layer of flattened cells connected joined by tight junction complexes. It has an important role in maintaining corneal transparency by active transport of water out of the stroma by Na/K-ATPase pumps. In humans, the corneal endothelial cells do not proliferate after birth and potential insult to this monolayer poses a risk for permanent corneal damage related to loss of function. Endothelial cell function may, for example, be compromised in diabetics where a decrease in the activity of the endothelial Na/K-ATPase pump has been demonstrated to cause functional and morphological changes (14). A significant decrease in endothelial cell density has been reported in diabetics (15) and this reduction is significantly greater in proliferative as compared to non-proliferative diabetic retinopathy patients (14). A recent systematic review and meta-analysis has shown that diabetics are associated with an increased risk of dry eye syndrome [odds ratio (OR): 1.15; P=0.019] (16) and therefore more likely to be investigated with topical corneal fluorescein staining.
There are a limited number of studies that have investigated the effects of fluorescein on corneal endothelium. Some of these studies assessed the effects of fluorescein on rabbit endothelial cells ex vivo using trans-endothelial electrical potential difference (17), and corneal endothelial morphology examined by non-contact specular microscopy (2). Although there are gross morphological similarities between rabbit and human corneas, the rabbit corneal endothelial cells have a different cytotoxic response compared to human cells, and rabbit corneal endothelial cells may proliferate after injury (17), while human corneal endothelium recovers by increased cell size and migration of endothelial cells (18).
The current study was conducted to evaluate the effects of the widely used ocular dye fluorescein in different concentrations and over a range of exposure times in an in vitro human corneal endothelial cell (HCEnC) culture model. To the best of our knowledge this is the first study to evaluate the effects of fluorescein in vitro in a HCEnC line. We present this article in accordance with the MDAR reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-24-22/rc).
Methods
Human endothelial serum free medium (SFM), mouse basic fibroblast growth factor (bFGF), TrypLETM Express and phosphate buffered saline (PBS) were purchased from Life Technologies, USA. Chondroitin sulfate and laminin were purchased from Sigma Aldrich, Australia. Alamar Blue was purchased from Thermo Fisher Scientific, Australia. Fluorescite 10% was purchased from Alcon Laboratories (Australia) Pty Ltd. (Macquorie Park, NSW, Australia). All the cell culture plastics were obtained from Corning, NY, USA.
Cell culture
B4G12, an immortalized human corneal endothelium cell line, a kind gift from Dr. M Valtink (Germany), was cultured according to a previous publication (19). In summary, cells were grown on a pre-coated tissue cultured flask (1 mg/mL chondroitin 6-sulphate and 10 µg/mL laminin for 1 hour, RT) with human endothelial-SFM supplemented with 10 ng/mL bFGF. Cells were passaged with TrypLETM Express when 90% confluence was reached. Passage 125 and 126 were used in this study.
Ethical approval was not required for this study because it did not involve human or animal participants, nor did it entail the use of personal data.
Fluorescein treatment
B4G12 cells were re-suspended with 150 µL culture medium and seeded on chondroitin 6-sulphate and laminin pre-coated 96 well plates at a density of 6×103 cells/well and 6 wells per treatment (n=6). When confluent, monolayers were treated with H2O (control diluent pH 9.0 adjusted by sodium hydroxide and hydrochloric acid), or fluorescein in a range of different concentrations (0.0001%, 0.001%, 0.01% and 0.05%) at various time points. The experiments were repeated four times for short-term exposure, and three times for long-term fluorescein exposure. Notably, the fluorescein concentrations used in this study were from dilutions Fluorescite 10% (pH 8.0–9.8) using culture medium.
Toxicity assessment
To assess cell viability, Alamar Blue assay was performed to detect cellular metabolic activity. B4G12 cells were resuspended with 150 µL growth media and seeded on chondroitin 6-sulphate and laminin pre-coated 96 well plates at a density of 6×103/well. When confluent, monolayers were incubated with Alamar Blue reagent according to the manufacturer’s protocol. Ten micro-liter of Alamar Blue reagent was added to 90 µL of freshly cultured medium in each well, followed by one hour of incubation at 37 ℃ as the baseline (0 time point). The resulting absorbance was read in a plate reader (Safire2 Tecan) at wavelengths 570–600 nm. The reagent was discarded, and the wells were gently washed with PBS once, and subsequently treated with either H2O (diluent, PH 9.0) or different concentrations of fluorescein for acute or continuous exposure as described below.
For the acute exposure, the monolayers were treated with either the growth medium containing 3 µL of H2O (total volume of 150 µL/well) or different concentrations of fluorescein (0.01%, 0.1% and 0.2%). The treatment was stopped at time-points of 1, 10, 30 and 60 minutes, and wells were gently washed with PBS twice. Subsequently 10 µL Alamar Blue reagent was added into each well that contained 90 µL of fresh culture medium, and the absorbance was read with a plate reader at 1, 2, 3 and 24 hours after treatment.
For the ‘chronic’ exposure, the monolayers were treated with either 0.75 µL of H2O (total volume of 150 µL/well) or different concentrations of fluorescein (0.0001%, 0.001%, 0.01% and 0.05%). The treatment was continued until day 4. The cell viability was assessed daily by Alamar Blue assay, followed by washing in PBS, and fresh treatments added. Cell morphology was observed daily by inverted phase-contrast microscopy, to evaluate the effects of fluorescein on HCEnCs.
Statistical analysis
Four experiments for short-term fluorescein exposure were conducted and three experiments for continuous long-term fluorescein exposure were performed; for both types of experiments six replicates per treatment group were used. Results were expressed as mean ± standard error of the mean (SEM). The statistical significance of the data was assessed using GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA). A two-way analysis of variance (ANOVA) was employed to evaluate the differences among groups. The choice of ANOVA is justified as it allows for the comparison of means across multiple groups and times, which is essential for our experimental design. A P value of <0.05 was considered statistically significant. Post-hoc analysis was performed using Tukey’s multiple comparisons test to account for multiple comparisons, ensuring that the increased risk of type I error was minimized. P values were reported according to the guidelines: if 0.001≤P<0.01, specific P values were reported to three decimal places.
Results
Short-term fluorescein exposure did not affect HCEnC viability
We first investigated if short-term fluorescein treatment affected HCEnC viability with Alamar Blue assay. The cell metabolic activity did not change when cells were exposed to fluorescein 0.01%, 0.1% and 0.2% at 1-, 10- and 30-minute durations, compared to the control. This indicated the cells were viable and healthy (Figure 1A, A1-A6), with no effects of cell recovery in fresh medium.
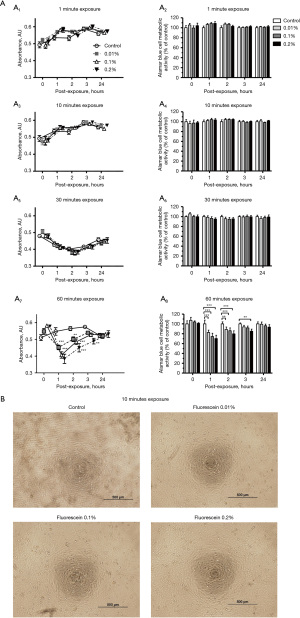
However, Alamar Blue assay results showed a significant reduction in cell metabolism following 60 minutes of fluorescein exposure. Specifically, cell metabolic activity was significantly inhibited 1-hour post-exposure to 0.01% (P<0.001), 0.1% (P<0.001) and 0.2% (P<0.001) of fluorescein, compared to the control (Figure 1A, A7 & A8). These results suggested that the cells were in an unhealthy state. Metabolic activity could be recovered after replacing cells in fresh culture medium for 24 hours. Cells previously exposed to 0.01% fluorescein still exhibited significantly lower metabolic activity than the control at 2 hours post-exposure (P=0.005). Moreover, metabolic activity in cells exposed to 0.1% and 0.2% fluorescein at this time point remained significantly lower (P=0.001 for both concentrations). Notably, the metabolic suppression observed at 0.2% fluorescein persisted even after 3 hours of exposure (P=0.001). These finding suggest that exposure to higher fluorescein concentrations delayed the recovery of cellular metabolic activity (Figure 1A, A7 & A8).
Morphological observations with phase contrast microscopy were consistent with the Alamar Blue assay. The cells exhibited a similar morphology after 10 minutes of fluorescein exposure with concentrations of 0.01%, 0.1% and 0.2% (Figure 1B).
Continuous fluorescein exposure greatly impaired the viability of HCEnCs
We next evaluated the impact of continuous fluorescein exposure on HCEnC viability (Figure 2A,2B). Cells exposed to 0.0001% fluorescein maintained metabolic activity and morphology comparable to the control for up to 4 days. However, exposure to 0.001% fluorescein markedly reduced metabolic activity, with a significant decrease observed after just 1 day (P<0.001), and a reduction to 50% of the control level after 4 days. Higher concentrations of fluorescein (0.01% and 0.05%) further reduced metabolic activity; and cells exposed to 0.05% fluorescein showed no detectable activity from day 2 onwards (Alamar Blue assay). In addition, cell metabolic activity was almost undetectable in the 0.01% group by day 3 (Figure 2A,2B).
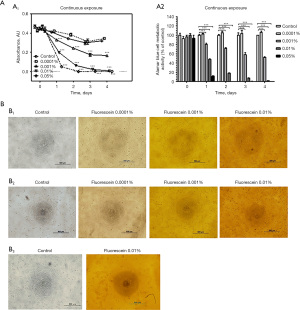
Cell morphology observations confirmed these findings (Figure 2B). No obvious differences in cell morphology were observed between the control and cells exposed to 0.0001% fluorescein throughout the experiment. In contrast, the 0.001% fluorescein exposure group showed reduced in cell density by day 2. On the other hand, for 0.01% fluorescein exposure many rounded cells were observed by day 1, with obvious cell death by day 2 indicated by cell shrinkage and detachment from the cell culture plastic (Figure 2B, B1 & B2). By day 3, many cells were detached and floating in the medium following continuous 0.01% fluorescein exposure (Figure 2B, B3).
Discussion
Our key findings demonstrated that short-term exposure to fluorescein in concentrations commonly used in eye drops does not affect HCEnC viability. No change in cell metabolic activity was observed for all fluorescein concentrations tested in this study (0.01–0.2%) for up to 30 minutes of exposure. Furthermore, cell metabolic activity was decreased when cells were exposed to fluorescein for 60 minutes for all concentrations tested, with a period of cell metabolic activity recovery to control levels at 24 hours. On the other hand, continued fluorescein exposure for more than 1 day significantly impaired the viability of HCEnCs. Cell metabolic activity was markedly reduced when cells were treated with fluorescein concentrations of 0.001–0.05% for more than 1 day. Cell viability decreased obviously with continued exposure until day 4, with higher fluorescein concentrations being associated with lower metabolic activity.
The main strength of this study is that it is the first reported in vitro usage of a human endothelial cell line to assess the effects of fluorescein on corneal endothelium. A human model better represents corneal endothelial cell responses to fluorescein compared to studies performed in other species. However, being an in vitro study, one of the limitations includes the different pharmacokinetics with respect to exposure time, dilution, and end-concentration of the fluorescein. Additionally, inflammatory responses were not simulated in this study, and tissue and systemic responses may be different from the cell responses in vitro.
There are reports of fluorescein toxicity on retinal neuronal growth (20), peripheral and central nervous system (21,22) and coordination of gait (7,23). Our study showed no cytotoxicity associated with acute exposure to fluorescein which is consistent with the findings of previous studies on the effect of fluorescein on rabbit corneal endothelial cells. Calli and colleagues (2) found fluorescein to have a wider therapeutic window compared to other dyes tested and suggested that one-minute exposure to fluorescein appeared to be safe as determined by no cytotoxic effects on rabbit corneal endothelial cells in culture. Similarly, Akiyama and colleagues (17) showed that no toxic effect of fluorescein was discernible at concentrations relevant to ophthalmic practice by utilising trans-endothelial electrical potential difference as a marker for cell function in the in vitro rabbit corneal endothelial cells. They concluded that the endothelial cell function decreased below control values after four hours of exposure to fluorescein at the concentration of 500 µg/mL.
The results of our study suggest that short-term fluorescein exposure in concentrations commonly used for the topical ophthalmic administration is safe and does not cause toxicity to corneal endothelial cells. However continued exposure may be associated with endothelial cytotoxicity. Although in routine practice the cornea does not tend to be exposed to fluorescein for long periods, our finding becomes more significant when the pharmacokinetic model behind fluorescein transport (including both paracellular and transcellular routes) as discussed below is considered. Furthermore, patient factors including dry eye, trauma, keratitis and aging, which induce epithelial defects, contribute to an increased concentration of fluorescein within the stroma and corneal endothelium during repetitive ophthalmic examinations. Fluorescein has also been used intraocularly for capsulorhexis (3), and it may be important for clinicians to ensure they have removed all fluorescein from the anterior chamber in these situations. Following fluorescein angiography in diabetics with both non-proliferative and proliferative retinopathy, no effects on corneal endothelial cell density, variation of cell area, average cell area, percentage of hexagonal cells and central corneal thickness at 1 hour, day 1, week 1 and 1-month post-angiography (1,2).
Fluorescein eye drops, like other topically administered eye drops, have a brief residence time with a half-life of approximately four minutes on the ocular surface, during which fluorescein can access the anterior chamber mainly by penetration across the cornea (24). Gupta and colleagues (12) showed that that apart from traversing through the paracellular route, fluorescein is transported across by the transcellular route, leading to a persistent increase in concentration of the dye in the endothelium.
Factors including dry eye disease and aging can further contribute to an increase in fluorescein concentration in the corneal endothelium. Fahim et al. (25) found that patients with dry eye disease have a 5-fold increase in corneal tissue fluorescein concentrations compared with normal subjects ten minutes after fluorescein administration. Thiel and colleagues (26) suggested that compromise of the epithelial barrier results in a 200-fold increase in penetration of fluorescein into the anterior chamber. Finally, Carlson and colleagues (27) concluded that with age, the human corneal endothelium becomes morphologically less regular and may become more permeable to fluorescein. Our in vitro study did not account for these risk factors and assumed that fluorescein concentration within the endothelium at different time points remained stable. However, based on the findings that increased exposure time to fluorescein was associated with increased risk of endothelial cytotoxicity, further research is needed in this area.
Conclusions
In summary, our study suggests that short-term exposure to fluorescein in concentrations commonly used in ophthalmic practice does not affect the corneal endothelium cell viability. Future studies could utilise an in vivo model and/or a primary corneal endothelial cell line to provide a better representation of corneal endothelial cells. These could be followed by ex vivo models. In vivo studies would aim to assess the effects of longer-term exposure to fluorescein on corneal endothelial function particularly in patients with risk factors such as dry eye disease, trauma, keratitis and aging, which induce epithelial defects.
Acknowledgments
We thank Dr. Monika Valtink for providing B4G12 cell line, Prof. Francis Billson and Prof. Kathy McClellan for their support and encouragement.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://aes.amegroups.com/article/view/10.21037/aes-24-22/rc
Data Sharing Statement: Available at https://aes.amegroups.com/article/view/10.21037/aes-24-22/dss
Peer Review File: Available at https://aes.amegroups.com/article/view/10.21037/aes-24-22/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-24-22/coif). K.G.O. serves as an unpaid editorial board member of Annals of Eye Science from November 2024 to December 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was not required for this study because it did not involve human or animal participants, nor did it entail the use of personal data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Limon U, Özsoy Saygın I, Gezginaslan TA, et al. Effects of fluorescein on corneal endothelial morphology after fundus fluorescein angiography in patients with diabetic macular edema. Cutan Ocul Toxicol 2023;42:91-6. [Crossref] [PubMed]
- Calli U, Ozturk Y, Demir G. The Effect of Fluorosecein on Corneal Endothelial Structure and Morphology in Diabetic Retinopathy Patients undergone Fundus Fluoresecein Angiography. Beyoglu Eye J 2022;7:35-8. [PubMed]
- Chang YS, Tseng SY, Tseng SH, et al. Comparison of dyes for cataract surgery. Part 1: cytotoxicity to corneal endothelial cells in a rabbit model. J Cataract Refract Surg 2005;31:792-8. [Crossref] [PubMed]
- Pavlopoulos GP, Giannakos GI, Theodosiadis PG, et al. Rubeola keratitis: a photographic study of corneal lesions. Cornea 2008;27:411-6. [Crossref] [PubMed]
- Fonn D, Peterson R, Woods C. Corneal staining as a response to contact lens wear. Eye Contact Lens 2010;36:318-21. [Crossref] [PubMed]
- Korb DR. Survey of preferred tests for diagnosis of the tear film and dry eye. Cornea 2000;19:483-6. [Crossref] [PubMed]
- Sindt CW, Critser DB, Grout TK, et al. Effects of fluorescein staining on laser in vivo confocal microscopy images of the cornea. J Ophthalmol 2012;2012:541974. [Crossref] [PubMed]
- Lindén C, Alm A. Effect of consecutively applied fluorescein eye drops on corneal and aqueous concentrations of fluorescein. Ophthalmic Res 1997;29:57-60. [Crossref] [PubMed]
- Sher I, Tzameret A, Goldberg Z, et al. Repetitive magnetic stimulation protects corneal epithelium in a rabbit model of short-term exposure keratopathy. Ocul Surf 2020;18:64-73. [Crossref] [PubMed]
- Alford R, Simpson HM, Duberman J, et al. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging 2009;8:341-54. [Crossref] [PubMed]
- Begum G, Leigh T, Courtie E, et al. Rapid assessment of ocular drug delivery in a novel ex vivo corneal model. Sci Rep 2020;10:11754. [Crossref] [PubMed]
- Gupta C, Chauhan A, Srinivas SP. Penetration of fluorescein across the rabbit cornea from the endothelial surface. Pharm Res 2012;29:3325-34. [Crossref] [PubMed]
- Shiraya K, Nagataki S. Movement of fluorescein monoglucuronide in the rabbit cornea. Diffusion in the stroma and endothelial permeability. Invest Ophthalmol Vis Sci 1986;27:24-28. [PubMed]
- Çolak S, Kazanci B, Ozçelik Soba D, et al. Effects of diabetes duration and HgA1C level on corneal endothelial morphology. Eur J Ophthalmol 2021;31:967-75. [Crossref] [PubMed]
- Weston BC, Bourne WM, Polse KA, et al. Corneal hydration control in diabetes mellitus. Invest Ophthalmol Vis Sci 1995;36:586-95. [PubMed]
- Qian L, Wei W. Identified risk factors for dry eye syndrome: A systematic review and meta-analysis. PLoS One 2022;17:e0271267. [Crossref] [PubMed]
- Akiyama R, Koniarek JP, Fischbarg J. Effect of fluorescein on the electrical potential difference across isolated rabbit corneal endothelium. Invest Ophthalmol Vis Sci 1990;31:2593-5. [PubMed]
- Bourne WM, Kaufman HE. Endothelial damage associated with intraocular lenses. Am J Ophthalmol 1976;81:482-5. [Crossref] [PubMed]
- Valtink M, Gruschwitz R, Funk RH, et al. Two clonal cell lines of immortalized human corneal endothelial cells show either differentiated or precursor cell characteristics. Cells Tissues Organs 2008;187:286-94. [Crossref] [PubMed]
- Kato S, Madachi-Yamamoto S, Hayashi Y, et al. Effect of sodium fluorescein on neurite outgrowth from the retinal explant culture: an in vitro model for retinal toxicity. Brain Res 1983;313:143-7. [Crossref] [PubMed]
- Pouliquen H, Algoet M, Buchet V, et al. Acute toxicity of fluorescein to turbot (Scophthalmus maximus). Vet Hum Toxicol 1995;37:527-9. [PubMed]
- Placantonakis DG, Tabaee A, Anand VK, et al. Safety of low-dose intrathecal fluorescein in endoscopic cranial base surgery. Neurosurgery 2007;61:161-5; discussion 165-6. [PubMed]
- Yankell SL, Loux JJ. Acute toxicity testing of erythrosine and sodium fluorescein in mice and rats. J Periodontol 1977;48:228-31. [Crossref] [PubMed]
- Gaudana R, Ananthula HK, Parenky A, et al. Ocular drug delivery. AAPS J 2010;12:348-60. [Crossref] [PubMed]
- Fahim MM, Haji S, Koonapareddy CV, et al. Fluorophotometry as a diagnostic tool for the evaluation of dry eye disease. BMC Ophthalmol 2006;6:20. [Crossref] [PubMed]
- Thiel MA, Morlet N, Schulz D, et al. A simple corneal perfusion chamber for drug penetration and toxicity studies. Br J Ophthalmol 2001;85:450-3. [Crossref] [PubMed]
- Carlson KH, Bourne WM, McLaren JW, et al. Variations in human corneal endothelial cell morphology and permeability to fluorescein with age. Exp Eye Res 1988;47:27-41. [Crossref] [PubMed]
Cite this article as: Wen L, Eghtedari Y, Ooi KG, Madigan MC, Watson SL, Petsoglou C, Bosch MM. The in vitro effect of fluorescein exposure on human corneal endothelial cells. Ann Eye Sci 2025;10:6.


