
Artificial intelligence and refractive surgeries including laser vision correction and phakic IOL implantation—a narrative review
Introduction
Background
Refractive errors, particularly myopia, have emerged as a significant global public health concern due to their rapidly increasing prevalence, especially among younger populations. Research estimates that by 2050, approximately 50% of the global population will be affected by myopia, with 10% experiencing high myopia (1). This trend is especially pronounced in East Asia, where the prevalence is increasing at an alarming rate (2). Myopia significantly affects vision-related quality of life, creating challenges in education, employment, and daily life (3). Consequently, these trends have driven a growing demand for effective vision correction solutions, including refractive surgeries.
Refractive surgery aims to correct refractive errors, such as myopia, hyperopia, and astigmatism, by reshaping the cornea or implanting corrective intraocular lenses (IOLs) (4). These procedures have significantly enhanced the quality of life for individuals reliant on glasses or contact lenses by offering clear, unaided vision (5). Laser vision correction (LVC) techniques have evolved over several decades, offering patients rapid visual recovery, improved precision, and reduced risks of complications. Additionally, phakic IOL implantation has emerged as an alternative for patients with high refractive errors or contraindications to corneal laser surgery (6). Despite their success, refractive surgery outcomes rely on surgical expertise, thorough preoperative evaluation, precise planning, and diligent postoperative care, all of which are complex and time-intensive (7).
Artificial intelligence (AI) has revolutionized ophthalmology by enabling more accurate, efficient, and personalized approaches to patient care (8). In refractive surgery, AI is increasingly integrated into various stages of clinical decision-making and surgical planning. AI-powered algorithms can analyze large volumes of data from imaging modalities such as corneal topography, anterior segment optical coherence tomography (OCT), and Scheimpflug imaging to identify subtle risk factors and optimize surgical outcomes (9,10). Machine learning models in medicine have been used to assess patient suitability, optimize surgical parameters, and predict postoperative outcomes (11). Multimodal AI systems, integrating data from imaging and clinical parameters, will enhance precision and consistency, improving patient management (12). Continued advancements in AI are anticipated to further transform refractive surgeries for vision correction.
Rationale and knowledge gap
Despite significant advancements in refractive surgery, there remain critical knowledge gaps that need to be addressed. Current approaches to patient selection, surgical planning, and outcome prediction rely heavily on clinician expertise and traditional nomograms, which can be subjective and prone to variability. While data-driven AI has shown promise in enhancing these processes (13,14), its application remains inconsistent, with limited consensus on standardized models and protocols. Furthermore, many studies focus on isolated components of refractive surgery, such as imaging or specific parameters, rather than harnessing the full potential of multimodal data integration (15,16). Expanding the understanding of how AI can improve outcomes across diverse patient populations and surgical techniques is essential. This review aims to bridge these gaps by synthesizing current evidence and identifying key areas for future research in AI applications for refractive surgeries.
Objective
The objective of this narrative review is to explore the integration of AI in refractive surgery, specifically in LVC and phakic IOL implantation, focusing on its impact on surgical outcomes. This review highlights how AI enhances patient selection, surgical planning, and postoperative prediction while addressing existing challenges and limitations. It also examines the role of AI-driven data analysis, including tabular, imaging, and multimodal data, in improving decision-making and surgical precision. By identifying key advancements and gaps in knowledge, this review provides insights into future opportunities for optimizing AI applications in refractive surgery. We present this article in accordance with the Narrative Review reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-24-40/rc).
Methods
This study utilized a general narrative review methodology to explore the integration of AI in refractive surgery, with a focus on LVC and phakic IOL implantation. The detailed study protocol is shown in Table 1. The literature search was conducted across multiple databases, including Google Scholar, PubMed, Embase, and Scopus, covering studies published from Jan 2010 to Oct 2024. The 2010 cutoff was selected as machine learning and deep learning advancements emerged after this period, with no relevant AI studies in refractive surgery found earlier. This exclusion ensured relevance and streamlined the review process. To ensure a thorough review, predefined Medical Subject Headings (MeSH) terms (“artificial intelligence” and “LASIK”) and free-text keywords including “artificial intelligence”, “machine learning”, “deep learning”, “refractive surgery”, “laser vision correction”, “phakic IOL”, “ICL”, “LASIK”, “PRK”, “ReLEx”, “SMILE”, “prediction”, “nomogram”, and “optimization” were used. Boolean operators were used to combine these terms systematically. Additionally, filters were applied to include only peer-reviewed articles published in English.
Table 1
| Items | Specification |
|---|---|
| Date of search | 5th November, 2024 |
| Databases and other sources searched | Google Scholar, PubMed, Embase, and Scopus |
| Search terms used | “Artificial intelligence”, “machine learning”, “deep learning”, “refractive surgery”, “laser vision correction”, “phakic IOL”, “ICL”, “LASIK”, “PRK”, “ReLEx”, “SMILE”, “prediction”, “nomogram”, and “optimization” |
| Timeframe | Jan 2010 to Oct 2024 |
| Inclusion and exclusion criteria | Inclusion: original research articles, peer-reviewed articles published in English, studies examining AI applications in refractive surgery |
| Exclusion: review papers, editorial articles, perspective articles, non-peer-reviewed conference abstracts | |
| Selection process | Titles and abstracts of all retrieved studies were screened independently by two reviewers to determine relevance. Full-text articles of potentially eligible studies were reviewed. Discrepancies were resolved through discussion or consultation with a third reviewer |
| Additional considerations | The review focused on studies exploring AI’s role in optimizing patient selection, enhancing surgical precision, and predicting postoperative outcomes |
AI, artificial intelligence; ICL, implantable Collamer lens; IOL, intraocular lens; LASIK, laser-assisted in situ keratomileusis; PRK, photorefractive keratectomy; ReLEx, refractive lenticule extraction; SMILE, small incision lenticule extraction.
The inclusion criteria were designed to capture studies that examined AI applications in refractive surgery, specifically those addressing patient selection, surgical planning, or postoperative outcome prediction. Articles were included if they presented original data or described clinically relevant AI methodologies applicable to LVC or phakic IOL implantation. Excluded articles comprised review papers, editorial articles, perspective articles, non-peer-reviewed conference abstracts, and studies focusing solely on experimental AI techniques with no clinical applicability. Additionally, studies unrelated to refractive surgery or AI integration were excluded.
Titles and abstracts of all retrieved studies were screened independently by two reviewers to assess relevance. Full-text articles deemed potentially eligible were subsequently reviewed, with discrepancies resolved through discussion or consultation with a third reviewer. Data extraction was performed systematically, capturing information on study design, AI methodologies employed, surgical techniques targeted, reported outcomes, and any limitations or ethical considerations noted in the studies. Key areas of focus included the role of AI in optimizing patient selection, improving surgical precision, and predicting postoperative outcomes.
The findings were synthesized narratively, emphasizing the current role of AI in refractive surgery and its potential to enhance surgical outcomes. Particular attention was given to the comparison of AI-driven approaches with traditional methods, as well as innovations in multimodal AI systems and real-time clinical decision support. Finally, a total of 33 studies (16 related to LVC and 17 focused on phakic IOLs) that met the inclusion criteria were collected and analyzed for their content. Additionally, challenges related to data variability, technical limitations, and ethical concerns were analyzed to provide a balanced perspective on the adoption of AI in refractive surgery. This review aimed to provide insights into how AI is transforming refractive surgery and to identify opportunities for future research and clinical application.
Types of refractive surgeries
Understanding the distinct characteristics of each refractive surgery is crucial for developing AI models optimized for specific procedures. AI applications must account for variations in corneal biomechanics, healing responses, and optical changes unique to each surgical technique. Refractive surgery comprises several procedural categories (4), each of which aims to correct refractive errors by altering either the corneal curvature or introducing an additional lens into the eye. Among these, LVC techniques and phakic IOL implantation represent two primary avenues for achieving improved refractive outcomes (Figure 1).
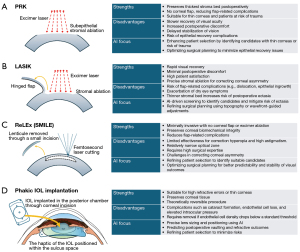
LVC
LVC encompasses a range of procedures, including photorefractive keratectomy (PRK), laser-assisted in situ keratomileusis (LASIK), and refractive lenticule extraction (ReLEx), that are designed to reshape the cornea and precisely modify its refractive power (17). AI has substantially influenced LVC by refining patient selection to avoid keratoconus, optimizing preoperative planning, and enhancing postoperative forecasting (13).
PRK removes the superficial corneal epithelium to allow excimer laser ablation, preserving the thickest stromal bed postoperatively, making it suitable for patients with high myopia or astigmatism. Unlike LASIK, it avoids flap-related complications, making it preferable for patients with thin corneas or those at risk of corneal trauma. However, PRK has slower visual recovery, greater postoperative discomfort, and delayed stabilization compared to other LVC techniques (4). AI has been explored in detecting corneal haziness using anterior segment OCT after PRK, aiding in postoperative monitoring and management (18).
LASIK involves creating a corneal flap with a microkeratome or femtosecond laser, followed by excimer laser ablation. It offers rapid visual recovery and minimal discomfort but carries risks such as flap dislocation, epithelial ingrowth, and worsening dry eye symptoms (4,19). The creation of the corneal flap also results in a thinner stromal bed, necessitating careful patient selection to minimize the risk of postoperative ectasia (20,21). AI-driven predictive models optimize screening and surgical planning, improving patient safety and reducing complications (20).
ReLEx [or Small Incision Lenticule Extraction (SMILE)], a minimally invasive alternative, creates an intrastromal lenticule with a femtosecond laser, which is removed through a small incision. This preserves corneal biomechanics and reduces flap-related complications (22). However, ReLEx has limitations in correcting hyperopia and high astigmatism and requires significant surgical expertise for consistent results (23). Additional drawbacks include a relatively narrow optical zone and the current inability to effectively address corneal asymmetry (24). AI plays a crucial role in enhancing patient selection, refining surgical planning, and improving predictability, ultimately contributing to more stable visual outcomes (21). Given that postoperative undercorrection is a common concern, several studies have explored AI-driven SMILE nomograms to optimize refractive accuracy (25,26).
Phakic IOL implantation
Phakic IOL implantation, particularly implantable Collamer lens (ICL) implantation, involves placing an artificial lens in the posterior chamber while preserving the natural crystalline lens. Iris-fixated anterior chamber IOLs are now rarely used due to complications like accelerated endothelial cell loss (27), while posterior chamber phakic IOLs have gained prominence. Innovations such as central-hole IOL designs have simplified surgery and reduced complications (28). If adverse events occur or corneal endothelial cell density drops below 1,500 cells/mm2 or declines by more than 30%, the risk of endothelial decompensation increases (29).
Posterior chamber phakic IOLs are ideal for patients with high refractive errors or thin corneas unsuitable for laser surgery. They offer broad refractive correction, preserve corneal integrity, and are theoretically reversible. However, potential complications include cataract formation, endothelial cell loss, and elevated intraocular pressure (30). Proper postoperative IOL vaulting is critical for long-term stability (31). AI enhances phakic IOL procedures by optimizing lens sizing, predicting postoperative vaulting (9), postoperative anterior chamber angle (32), and accurately forecasting refractive outcomes (33). These advancements improve surgical precision, reduce complications, and refine patient selection, ensuring safer and more effective outcomes.
Data types for refractive surgery
The application of AI in refractive surgery relies on the successful integration of multiple data types, each requiring distinct analytical approaches. Generally, tabular data, image data, and multimodal data inform various stages of the patient care process in various medical fields, from initial risk assessment to postoperative follow-up (34,35), and AI-driven models have the capacity to optimize decision-making in all these areas (Figure 2).

Tabular data
Tabular data include numerical and categorical variables arranged in structured formats. Relevant examples encompass manifest refraction values, keratometry readings, pupil size measurements, and white-to-white distances. Traditional machine learning algorithms, such as linear regression models, support vector machines, random forests, and gradient boosting, are commonly applied to these datasets (36). Their strength lies in detecting and quantifying correlations and patterns that guide patient selection, evaluate surgical feasibility, estimate postoperative refraction, and refine nomograms. When systematically integrated, tabular data-driven models can offer personalized recommendations that help surgeons predict surgical outcomes with greater confidence. For example, refractive power and corneal curvature data can be structured into a table and analyzed to determine the most suitable refractive surgery procedure (13).
Image data
Image data, derived from modalities such as corneal topography, anterior segment OCT, and ultrasound biomicroscopy (UBM), contribute critical insights into ocular morphology and tissue integrity. Advanced deep learning architectures—particularly convolutional neural networks (CNNs) and vision transformers (ViTs)—are employed to interpret these visual datasets (37). Such AI models excel at recognizing subtle morphological features that might signal early keratoconus, irregular astigmatism, or corneal scarring. By leveraging large, annotated image repositories, these algorithms can detect abnormalities that inform surgical planning and improve safety, allowing clinicians to anticipate complications and tailor interventions more precisely. For example, it can be used to directly analyze corneal images or fundus images in preoperative evaluation. For instance, CNNs can directly analyze corneal or fundus images during preoperative evaluations to optimize patient selection and risk assessment (38,39).
Multimodal data
Multimodal data combine tabular parameters with image-based findings and may also integrate textual notes and measurements derived from advanced diagnostic devices such as Pentacam, Galilei, and IOL Master. AI techniques, including optical character recognition (OCR) for data extraction, object detection, and specialized multimodal models, enable cohesive analysis that synthesizes diverse information streams. By simultaneously analyzing numeric values, structural images, and device-specific metrics from refractive error data and corneal imaging, these systems uncover subtle correlations that might be overlooked when relying on a single data source (20). Recent advancements in multimodal AI chatbots enable them to recognize input image types, isolate and analyze relevant regions, extract numerical data, and generate clinically actionable insights. This not only streamlines clinical workflows but also enhances patient assessment accuracy, directly influencing decision-making and potentially improving surgical outcomes.
AI tasks for refractive surgery
AI applications in refractive surgery address critical aspects of patient care, encompassing classification, regression, data generation, and text generation. Classification tasks involve categorizing data into predefined groups, such as identifying suitable candidates for specific surgical procedures or detecting conditions like keratoconus, thereby enhancing patient selection and risk stratification through precise, data-driven categorizations (13,38). Selecting the optimal surgical method for a patient is also a classification problem (21). Regression models predict continuous outcomes, such as postoperative refractive error, nomogram, or ICL vault, enabling personalized surgical planning and optimizing procedural outcomes (40,41). Data generation leverages advanced AI techniques, including generative models like variational autoencoder, generative adversarial nets, and diffusion approaches, to create synthetic datasets that supplement limited real-world data, improving model robustness and broadening AI applicability in refractive surgery (42,43). For example, the predictive accuracy of CNN-based ICL vault estimation can be enhanced by generating synthetic images using generative techniques (44). Additionally, AI-powered large language models (LLMs) facilitate text generation by processing and producing descriptive or explanatory content, offering clinical insights such as patient-specific recommendations, surgical strategies, or postoperative counseling notes (45). With reasoning capabilities and attention mechanisms, they enhance communication, decision-making, and workflow efficiency. LLMs also process unstructured data and enable simple tool development, such as calculators for refractive surgery and clinical practice (20,46). Collectively, these AI tasks, when integrated with the diverse data types used in refractive surgery, contribute to greater precision, improved safety, and more effective patient management, ultimately advancing the field and improving clinical outcomes.
Specific tasks in AI for refractive surgery
Literature review
The application of AI in refractive surgery has demonstrated transformative potential across multiple domains. As summarized in Table 2, machine learning techniques, such as extreme gradient boosting (XGBoost), ensemble models, and random forests applied to tabular data (e.g., ocular biometry and Pentacam metrics) have significantly advanced candidate selection (13,38), optimization (25,26), postoperative risk or outcome prediction for phakic IOLs (40,41) and LVC based on biomechanical (49) and topographic data (47,48), as well as surgical method selection (21,45,50). In LVC, ML models have outperformed traditional tools like the percentage tissue altered (PTA), residual stromal bed (RSB), and Randleman ectasia score system (13). Deep learning models, including CNNs (e.g., Inception-ResNetV2, ResNet50) and variational autoencoder-based techniques, have shown exceptional accuracy in detecting keratoconus (42), identifying high-risk corneas (38), and assessing corneal haze (18). Multimodal AI approaches, combining tabular and image data, have been particularly effective in predicting ectasia and myopic regression risks (39,47), while tools like ChatGPT-4 demonstrate potential as preliminary categorizers for procedure and candidate selection.
Table 2
| Publication | Category | Study design | AI technique | Domain | Target | Summary |
|---|---|---|---|---|---|---|
| Achiron et al., 2017 (14) | Prognosis prediction | Retrospective study for model development and validation | Machine learning (XGBoost) | Tabular data (ocular biometry and surgery parameter data) | Prediction of postoperative visual acuity | The model can assist in clinical decision-making by improving individual risk assessment, enabling the identification of groups with high and low visual outcome efficacy |
| Lopes et al., 2018 (47) | Patient selection | Multicenter case-control study | Machine learning (Random Forest) | Tabular data (Pentacam data) | Detection of corneal ectasia susceptibility | The random forest improves ectasia diagnosis, but further integration with corneal biomechanical parameters and the impact of laser vision correction is necessary for comprehensive ectasia risk assessment |
| Yoo et al., 2019 (13) | Prognosis prediction | Retrospective study for model development and validation | Machine learning (ensemble of various techniques) | Tabular data (ocular biometry and Pentacam data) | Selection of corneal LVC surgery candidates | Machine learning models demonstrated statistically significant superiority over traditional methods, such as PTA and the Randleman score, for candidate selection |
| Xie et al., 2020 (38) | Patient selection | Diagnostic, cross-sectional retrospective study | CNN (Inception-ResNetV2) | Image data (Pentacam image) | Selection of corneal LVC surgery candidates | The deep learning model proved effective in classifying images to provide detailed corneal information and preliminarily identify corneas at risk |
| Cui et al., 2020 (25) | Optimization | Prospective, comparative clinical study | Machine learning (artificial neural network) | Tabular data (ocular biometry and treatment features) | Developing a nomogram for SMILE input values | The machine learning technique matched the surgeon’s performance in terms of safety but significantly outperformed the surgeon in efficacy |
| Yoo et al., 2020 (21) | Surgical method selection | Retrospective study for model development and validation | Machine learning (XGBoost) | Tabular data (ocular biometry and Pentacam data) | LVC method selection | Machine learning models achieved performance comparable to expert-level assessments when selecting appropriate refractive surgery options for patients |
| Santhiago et al., 2022 (48) | Patient selection | Comparative case-control study | Machine learning and t-SNE | Tabular data (PTA, corneal thickness, derivative PTA, and age weighted value) | Prediction of postoperative ectasia | The machine learning model effectively combined various risk factors to identify individuals at elevated risk for LVC |
| Kim et al., 2022 (39) | Prognosis prediction | Retrospective study for model development and validation | Machine learning (XGBoost) and CNN (ResNet50) | Tabular data (clinical data) and image data (fundus photography) | Prediction of postoperative myopic regression | The multimodal machine learning algorithm offered an effective approach for detecting patients at high risk of myopic regression |
| Ambrósio et al., 2023 (49) | Patient selection | Multicenter cross-sectional case-control retrospective study | Machine learning (Random Forest) | Tabular data (Pentacam and Corvis ST data) | Ectasia risk detection for LVC | AI-driven optimization that integrates Scheimpflug-based corneal tomography and biomechanical evaluations enhances the accuracy of ectasia detection |
| Ćirković et al., 2023 (45) | Surgical method selection | Retrospective study | ChatGPT-4 | Tabular data (ocular biometry and Pentacam data) | Selection of LVC candidates and method selection | ChatGPT-4 demonstrated potential as a preliminary categorization tool for candidate and procedure selection in refractive surgery, exhibiting strong agreement with clinician evaluations |
| Agharezaei et al., 2023 (42) | Patient selection | Case-control study for model development and validation | VAE and CNN | Image data (corneal topography image) | Screening keratoconus for refractive surgery | The VAE-based deep learning models demonstrated high accuracy in detecting keratoconus using corneal topography images |
| Luft et al., 2024 (26) | Optimization | Retrospective study for model development and validation | Machine learning (artificial neural network) | Tabular data (ocular biometry and treatment features) | Developing a nomogram for SMILE input values | Machine learning validated the effectiveness of advanced linear and non-linear SMILE nomograms |
| Li et al., 2024 (50) | Surgical method selection | Retrospective study for model development and validation | Machine learning | Tabular data (ocular biometry, Pentacam, and ocular wavefront aberrometer) | LVC method selection | Machine learning models accurately predicted the final selection of corneal refractive surgery techniques |
| Awwad et al., 2024 (18) | Postoperative evaluation | Prospective study for model evaluation | CNN (segmentation model) | Image data (anterior segment OCT) | Corneal haze and demarcation line detection in post-PRK | The machine learning model quantitatively demonstrated that combining CXL with PRK without MMC does not lead to increased haze compared to accelerated CXL alone |
AI, artificial intelligence; CNN, convolutional neural network; CXL, corneal cross-linking; ICL, implantable Collamer lens; LVC, laser vision correction; MMC, mitomycin C; OCT, optical coherence tomography; PRK, photorefractive keratectomy; PTA, percent tissue altered; SMILE, small incision lenticule extraction; VAE, variational autoencoder; XGBoost, extreme gradient boosting.
Similarly, Table 3 highlights the use of AI in phakic IOL lens (or ICL) implantation, where machine learning models, such as random forest and XGBoost, have surpassed traditional nomograms in predicting postoperative vault size and refractive outcomes (33,40,41,51-56,59,61). Deep learning techniques have effectively analyzed imaging data from anterior segment OCT and UBM, addressing challenges like imbalanced datasets (57,62). Innovations such as data-balancing methods and no-code custom nomograms have further enhanced AI’s ability to improve postoperative accuracy, and ensure safer procedures in each individual institution (58,60). Collectively, these findings emphasize the pivotal role of AI in improving outcomes in refractive surgery.
Table 3
| Publication | Task | Study design | AI technique | Domain | Target | Summary |
|---|---|---|---|---|---|---|
| Kamiya et al., 2021 (40) | Prognosis prediction & optimization | Retrospective study for model development and validation | Machine learning (Random Forest) | Tabular data (biometry data from anterior segment OCT) | Postoperative ICL vault prediction | Machine learning applied to preoperative OCT metrics demonstrated significantly greater predictability of the ICL vault compared to the conventional manufacturer’s nomogram |
| Kang et al., 2021 (51) | Prognosis prediction & optimization | Retrospective study for model development and validation | Machine learning (XGBoost and ensemble) | Tabular data (biometry data from AS OCT) | Postoperative ICL vault prediction and size selection | Utilizing an ensemble approach on a large dataset improved the accuracy of vault size predictions and the selection of the optimal ICL size |
| Shen et al., 2023 (52) | Prognosis prediction & optimization | Retrospective study for model development and validation | Machine learning (Random Forest and other techniques) | Tabular data (biometry data, Pentacam, UBM data) | Postoperative ICL vault prediction | Machine learning models can predict vault size and optimize ICL sizing, assisting ophthalmologists in improving clinical outcome prediction |
| Chen et al., 2023 (53) | Prognosis prediction & optimization | Retrospective, cross-sectional study for model development and validation | Machine learning (Ensemble) | Tabular data (Pentacam, Sirius, and UBM) | Postoperative ICL vault prediction | Strategies utilizing multiple machine learning algorithms tailored to various ophthalmic devices and combinations are effective for predicting vault size |
| Jiang et al., 2023 (41) | Prognosis prediction & optimization | Retrospective study for model development and validation | Machine learning (Ensemble) | Tabular data (biometry data and Pentacam) | Postoperative refractive error prediction | Machine learning models demonstrate comparable accuracy to existing methods while offering potential advantages in predicting postoperative refractive errors and calculating lens power |
| Choi et al., 2023 (32) | Prognosis prediction & optimization | Retrospective study for model development and validation | Machine learning (XGBoost) | Tabular data (biometry data from AS OCT) | Postoperative anterior chamber angle prediction | Machine learning algorithms enable surgeons to account for the postoperative anterior chamber angle, enhancing surgical accuracy and safety |
| Russo et al., 2023 (54) | Prognosis prediction & optimization | Retrospective multicenter comparison study | Machine learning (various techniques) | Tabular data (biometry data from AS OCT) | Postoperative ICL vault prediction | Machine learning demonstrated exceptional accuracy in predicting ICL vault and size, surpassing the performance of the manufacturer’s online nomogram |
| Chen et al., 2023 (55) | Prognosis prediction & optimization | Retrospective study for model validation | Machine learning (Random Forest) | Tabular data (biometry and Pentacam data) | Postoperative ICL vault prediction | The machine learning model optimized using sulcus-to-sulcus measurements outperformed the traditional white-to-white model in accurately predicting the vault |
| Jiang et al., 2024 (33) | Prognosis prediction & optimization | Retrospective study for model development and validation | Machine learning (Random Forest) | Tabular data (biometry data) | Postoperative refractive error (sphere and cylinder) prediction | The random forest-based calculator, integrating various ocular parameters, demonstrated superior performance over the existing calculator in predicting refractive errors on the study datasets |
| Zhu et al., 2024 (56) | Prognosis prediction & optimization | Retrospective study for model development and validation | Machine learning (voting with various techniques) | Tabular data (biometry data) | Postoperative ICL vault prediction | This study shows the potential of various machine learning algorithms to improve the prediction of postoperative vault and ICL size |
| Assaf et al., 2023 (57) | Postoperative evaluation | Retrospective, cross-sectional study for model development and validation | CNN (transfer learning-based) | Image data (AS OCT images) | Measurement of ICL vault | Deep learning effectively calculated the ICL vault from OCT scans, addressing challenges posed by imbalanced datasets and limited training data |
| Shin et al., 2024 (58) | Prognosis prediction & optimization | Two-center retrospective study | Machine learning (various techniques from a code-free tool) | Tabular data (biometry and Pentacam data) | Postoperative ICL vault prediction | Due to substantial variability in measurements and surgical practices across clinics, the no-code creation of a tailored machine learning nomogram can enhance the accuracy |
| Di et al., 2024 (59) | Prognosis prediction & optimization | Retrospective study for model development and validation | Machine learning (XGBoost) | Tabular data (biometry data from AS OCT) | Postoperative ICL vault prediction | The proposed machine learning model allows surgeons to account for the postoperative vault, thereby minimizing surgical complications |
| Zhao et al., 2024 (60) | Prognosis prediction & optimization | Retrospective study for model development and validation | Data-balancing machine learning | Tabular data (biometry data, AS OCT, and UBM) | Postoperative ICL vault prediction | The innovative data balancing-based machine learning model offers improved accuracy in predicting ICL size and postoperative vault |
| Assaf et al., 2024 (44) | Postoperative evaluation | Retrospective, cross-sectional study | Generative adversarial network and CNN | Image data (AS OCT images) | Measurement of ICL vault | The incorporation of GAN-generated and modified synthetic images significantly improved the accuracy of ICL vault estimation |
| Yang et al., 2024 (61) | Prognosis prediction & optimization | Retrospective study for model development and validation | CNN and various machine learning techniques | Tabular data (biometry data, AS OCT, and UBM) | Postoperative ICL vault prediction | A CNN-based model utilizing biometry data helps doctors select the optimal ICL size, enhancing the safety and postoperative outcomes of ICL surgery |
| Nasser et al., 2024 (62) | Prognosis prediction & optimization | Retrospective study for model development and validation | CNN | Image data (UBM images) | Postoperative ICL vault prediction | The deep learning model demonstrated high accuracy in predicting postoperative ICL vault, with most predictions falling within a clinically acceptable margin |
AS, anterior segment; ICL, implantable Collamer lens; IOL, intraocular lens; CNN, convolutional neural network; GAN, generative adversarial network; OCT, optical coherence tomography; UBM, ultrasound biomicroscopy; XGBoost, extreme gradient boosting.
The application of AI in refractive surgery can be categorized into four key areas: patient selection, surgical method determination, procedural optimization, and postoperative outcome prediction.
Patient selection for refractive surgery
The initial and perhaps most critical stage in refractive surgery involves determining patient eligibility. Traditionally, selection criteria are derived from established clinical guidelines that consider factors such as patient age, refractive stability, and ocular health. Contraindications, including active ocular surface disease, uncontrolled glaucoma, and certain degenerative corneal conditions, must be identified to prevent inappropriate treatment. Emerging AI-driven tools can augment this process, offering a more comprehensive risk assessment that accounts for subtle individual variations (63-65). Previously, patient screening relied on simple indicators like PTA, RSB, the Randleman Ectasia Risk Score, or subjective expert evaluations (16). AI enables a comprehensive, data-driven, quantitative approach, improving accuracy and consistency in assessing surgical candidacy (13).
Within the domain of LVC, advanced screening methodologies focus on detecting conditions that predispose patients to postoperative complications. For instance, screening for early signs of ectasia or postoperative disorders, including subclinical keratoconus, can be enhanced by AI models trained to analyze corneal topography, tomography, and biomechanical metrics (47,49). Similarly, corneal dystrophies and recurrent corneal erosions, which might jeopardize surgical outcomes, can be more accurately flagged through machine learning algorithms applied to high-resolution imaging datasets (66). In the context of ICL implantation, critical parameters such as anterior chamber depth, angles, and IOL vaulting can be evaluated with greater reliability and consistency using AI-enabled image interpretation, thereby guiding surgeons toward safer lens sizing and positioning (32,40,67).
General ocular comorbidities, particularly keratoconus, severe dry eye, or ocular surface irregularities, remain significant contraindications to refractive interventions. AI tools, bolstered by large-scale clinical databases, can efficiently synthesize patient history, clinical exam findings, and advanced imaging results to identify these conditions at earlier stages. High-fidelity imaging modalities, including OCT, Scheimpflug imaging, and the Corvis ST, provide rich datasets that inform AI-driven classification systems (68). As these systems become increasingly sophisticated, specialized algorithms can be developed for individual surgeons or institutions. Such customization ensures that patient selection criteria are tailored to unique patient populations and the surgeon’s specific expertise, ultimately reducing the incidence of postoperative complications and improving overall surgical success rates.
Advanced AI for surgical method selection
Selecting the appropriate surgical technique requires balancing the benefits and risks of different modalities, including LASIK, PRK, SMILE, and ICL implantation (45,50). AI-based risk assessment models can incorporate patient-specific data—ranging from detailed corneal imaging and higher-order aberration profiles to lifestyle factors and long-term visual needs—into predictive algorithms that suggest the most favorable intervention (21). Studies have demonstrated AI’s ability to objectively determine the optimal surgery for individual patients based on data (21,50).
Institutional and surgeon-specific AI tools can further refine this process by integrating historical outcomes data from their respective practices (58). By drawing upon a surgeon’s personal database of cases, algorithms can identify patterns that correlate with favorable results for specific patient subsets. In this manner, the AI models do not simply replicate the wisdom found in broad literature; they also localize and personalize decision-making, thereby enhancing precision, reducing intra-operative risk, and improving overall refractive outcomes when compared to traditional selection approaches.
Optimization of surgery through AI
The optimization of refractive surgical procedures has historically relied on manufacturer-based nomograms and empirical adjustments. Machine learning algorithms, trained on extensive surgical data, can refine SMILE nomograms (25,26), optimize treatment profiles, and adjust ICL lens sizing and positioning, demonstrating superior predictive accuracy over traditional nomograms (51,52,55). Additionally, machine learning models can be tailored to individual eye centers by leveraging their own surgical data repositories (58).
In LVC, AI-driven platforms optimize ablation profiles by integrating corneal topography, biomechanical strength, and wavefront aberrations, reducing complications such as induced aberrations and night vision disturbances (69). In ICL implantation, AI enhances surgical precision by predicting induced astigmatism, refining lens orientation, and optimizing lens size through vault prediction (33,40). Study has shown AI outperforms manufacturer calculators in determining the ideal IOL size and type for ICL surgery (9). These advancements standardize procedures, minimize variability, and improve overall surgical precision and patient outcomes.
Prediction of surgical prognosis with AI
AI-driven predictive modeling extends beyond surgery to long-term patient management. By synthesizing preoperative metrics, surgical details, and individual healing responses, AI can forecast postoperative visual acuity and refractive stability, helping clinicians anticipate outcomes and identify patients needing additional interventions (14,32,33,41).
AI also enhances complication monitoring by analyzing corneal maps and patient-reported symptoms to detect early signs of ectasia, dryness, or other ocular surface issues before they become significant (49). Predictive techniques using preoperative fundus photographs, surgical methods, and biometry data can assess the risk of postoperative myopic regression (39). In ICL management, AI improves accuracy in predicting postoperative vault and anterior chamber angle, aiding in reoperation decisions (32,60). Additionally, AI-derived prognostic models support patient counseling by providing evidence-based outcome predictions, improving understanding, expectation management, and overall satisfaction.
Postoperative measurement
AI has improved postoperative evaluation by enhancing accuracy in corneal haze detection after PRK and ICL vault measurement using anterior segment OCT images. A CNN-based segmentation model effectively identified corneal haze and demarcation lines in post-PRK patients (18). For ICL vault assessment, a CNN model with transfer learning accurately measured vault height, addressing challenges from imbalanced datasets and limited training data (57), while synthetic images further improved estimation precision (44). These advances demonstrate AI’s increasing role in improving postoperative assessments and refractive surgery outcomes.
Key finding and future of refractive surgery with multimodal AI
The key finding of this review is AI’s transformative impact on refractive surgery, improving patient selection, surgical planning, and postoperative management. Machine learning has enhanced ectasia risk assessment in LVC (13,38,49), optimized SMILE nomograms (25), and improved ICL vault prediction (40,54,55), while deep learning has demonstrated accuracy in postoperative corneal haze detection (18) and ICL vault measurement using OCT (44). AI-driven risk assessment tools have surpassed traditional nomograms in lens sizing and refractive outcome prediction, increasing precision and personalization (41,58). However, challenges such as single-modality reliance, data variability, model interpretability, and regulatory hurdles remain, necessitating further validation for widespread clinical adoption.
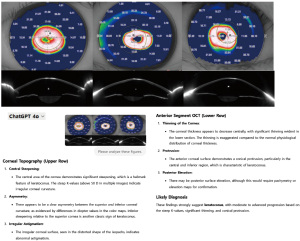
The integration of multimodal AI and LLMs is set to transform refractive surgery by combining structured clinical data, imaging, and patient-reported outcomes within unified analytical frameworks (35,70). AI-powered systems can merge clinical metrics—such as corneal topography, OCT, and wavefront aberrometry—to provide a comprehensive patient assessment, enhancing precision in surgical planning. A recent study demonstrated that multimodal LLMs can independently assess corneal characteristics, evaluate refractive power, and estimate surgical risk, enabling customized risk calculators for preoperative decision-making (20). Notably, multimodal LLMs now allow pre- and postoperative corneal evaluation without prior training. Figure 3 illustrates an example of corneal topography and anterior segment OCT imaging from a prior study (71), integrated with ChatGPT-4 analysis in our experiment. The AI successfully identified key diagnostic features for LVC, such as central steepening, thinning, and irregularity, which are indicative of keratoconus. These AI-driven corneal interpretations can aid in preoperative evaluations and risk factor assessments. Looking ahead, AI chatbots will simplify clinical application by enabling seamless interpretation of results, eliminating the need for complex machine learning interfaces, and making AI more accessible for real-world ophthalmic practice.
Beyond preoperative planning, AI-driven intraoperative guidance systems, including speaker and video-assisted technologies, offer significant benefits (72,73). During LASIK, SMILE, PRK, and phakic IOL implantation, AI provides real-time feedback, optimizes surgical parameters, and assists with ablation patterns, lens alignment, and incision placement, particularly when accessing information is challenging in sterile conditions (74). High-speed AI imaging analyzes live surgical feeds, highlights key landmarks, and detects subtle corneal or ocular tissue variations, enhancing precision and reducing errors. Voice recognition enables hands-free control, streamlining workflows and maintaining surgical focus. Additionally, AI-guided timeout systems verify critical steps, such as site preparation and equipment calibration, improving safety and consistency (75). These innovations enhance accuracy, efficiency, and patient outcomes in refractive surgery.
The role of AI in refractive surgery is set to expand, bringing unprecedented accuracy, personalization, and safety to surgical planning and execution. Advancements such as ray-tracing technology, next-generation laser devices like the VISUMAX800, and novel IOL designs (76-79) are rapidly broadening surgical options. AI-driven customization is reshaping refractive surgery by optimizing surgical selection, fine-tuning procedures, and enhancing clinical outcomes. As predictive models become more sophisticated, future AI innovations will enable personalized risk assessments, optimized surgical parameters, and precise long-term outcome predictions, improving patient satisfaction and success rates. While further technological advancements and regulatory alignment are needed (80), the integration of multimodal AI represents a transformative shift in refractive surgery, redefining vision correction through enhanced precision and patient-centered care.
Challenges and limitations of AI
Despite AI’s potential in refractive surgery, several challenges must be addressed to ensure safe, effective, and equitable integration. A key limitation is the variability in data across clinical settings (9,58). Differences in refraction, patient demographics, imaging modalities, and documentation standards create heterogeneous datasets, compromising the generalizability and reliability of AI models. To enhance adaptability and ensure consistent outcomes, rigorous external validation is essential during model development (81).
Data quality and availability also pose significant hurdles. AI models require large, well-annotated datasets, yet access is often restricted by privacy laws, limited inter-institutional collaborations, and incomplete electronic health records (82,83). Technical challenges include the risk of overfitting, deep learning interpretability issues, and difficulties in integrating diverse data types, all of which must be addressed to prevent AI tools from complicating clinical workflows (84). Developing transparent, interpretable models with clear reasoning pathways and refining multimodal data integration are key priorities (21,85).
Ensuring transparency and accountability is critical as clinicians increasingly rely on AI for surgical decisions. Collaboration among regulators, clinicians, and stakeholders is necessary to establish guidelines that balance innovation with patient safety and trust. While multimodal AI holds immense promise in refractive surgery, its success depends on effectively managing data diversity, technical complexity, ethical considerations, and regulatory compliance. Addressing these challenges will be crucial for AI to augment clinical expertise, improve patient outcomes, and advance the field.
Strengths and limitation of this review
This review provides a recent analysis of AI applications in refractive surgery, specifically in LVC and phakic IOL implantation. By integrating findings from machine learning and deep learning studies, it highlights AI’s role in patient selection, surgical planning, and postoperative prediction. A key strength of this review is its emphasis on clinical applicability, bridging the gap between AI advancements and real-world refractive surgery. Additionally, by addressing challenges such as data variability, model interpretability, and regulatory concerns, this review offers a balanced perspective on AI adoption and future research directions.
However, this review has certain limitations. As a narrative review, it does not follow a systematic or scoping methodology, which may introduce selection bias and limit reproducibility. Only English-language studies were included, potentially overlooking relevant research in other languages. Additionally, while the review discusses various AI models, it does not extensively cover newer techniques such as reinforcement learning or federated learning. Given the rapid evolution of AI in ophthalmology, some findings may become outdated, emphasizing the need for ongoing updates and systematic evaluations in future studies.
Conclusions
AI is revolutionizing refractive surgery by enhancing patient selection, optimizing surgical techniques, and improving postoperative management. By integrating clinical measurements and advanced imaging, AI models refine eligibility assessments, personalize surgical parameters, and predict long-term outcomes with greater accuracy than traditional methods. These advancements enable better risk stratification, customized treatment planning, and more informed patient counseling, ultimately improving satisfaction and safety.
Future multimodal AI systems, capable of analyzing imaging, text, clinical metrics, and patient-reported data, promise a comprehensive understanding of each patient’s unique profile. AI-driven algorithms tailored to local populations, surgeon-specific techniques, and individual anatomical variations will further enhance precision and effectiveness. Additionally, the integration of voice-activated controls, real-time feedback, and intraoperative AI guidance will create a more interactive and data-driven surgical environment.
The future of refractive surgery lies in seamlessly integrating AI with clinical expertise. By embedding AI into workflows, surgeons can leverage its capabilities to detect subtle patterns, refine decision-making, and enhance patient-centered care. This synergy will drive superior outcomes, improve efficiency, and redefine vision correction with greater accuracy and personalization.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aes.amegroups.com/article/view/10.21037/aes-24-40/rc
Peer Review File: Available at https://aes.amegroups.com/article/view/10.21037/aes-24-40/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-24-40/coif). T.K.Y. is a consultant for MediWhale and LuxMind. H.C. is an employee of B&VIIT Eye Center. Y.C. is a consultant for MediWhale. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016;123:1036-42. [Crossref] [PubMed]
- Surl D, Seo Y, Han J. Trends in myopia prevalence among late adolescents in South Korea: a population-level study and future projections up to 2050. BMJ Open Ophthalmol 2024;9:e001748. [Crossref] [PubMed]
- Takashima T, Yokoyama T, Futagami S, et al. The quality of life in patients with pathologic myopia. Jpn J Ophthalmol 2001;45:84-92. [Crossref] [PubMed]
- Kim TI, Alió Del Barrio JL, Wilkins M, et al. Refractive surgery. Lancet 2019;393:2085-98. [Crossref] [PubMed]
- Chen CY, Keeffe JE, Garoufalis P, et al. Vision-related quality of life comparison for emmetropes, myopes after refractive surgery, and myopes wearing spectacles or contact lenses. J Refract Surg 2007;23:752-9. [Crossref] [PubMed]
- Igarashi A, Shimizu K, Kamiya K. Eight-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am J Ophthalmol 2014;157:532-9.e1. [Crossref] [PubMed]
- Teus MA, de Benito-Llopis L, Sánchez-Pina JM. Learning curve of laser-assisted subepithelial keratectomy: influence on visual and refractive results. J Cataract Refract Surg 2007;33:1381-5. [Crossref] [PubMed]
- Choi JY, Yoo TK. New era after ChatGPT in ophthalmology: advances from data-based decision support to patient-centered generative artificial intelligence. Ann Transl Med 2023;11:337. [Crossref] [PubMed]
- Kim T, Kim SJ, Lee BY, et al. Development of an implantable collamer lens sizing model: a retrospective study using ANTERION swept-source optical coherence tomography and a literature review. BMC Ophthalmol 2023;23:59. [Crossref] [PubMed]
- Rampat R, Deshmukh R, Chen X, et al. Artificial Intelligence in Cornea, Refractive Surgery, and Cataract: Basic Principles, Clinical Applications, and Future Directions. Asia Pac J Ophthalmol (Phila) 2021;10:268-81. [Crossref] [PubMed]
- Elfanagely O, Toyoda Y, Othman S, et al. Machine Learning and Surgical Outcomes Prediction: A Systematic Review. J Surg Res 2021;264:346-61. [Crossref] [PubMed]
- Fetit AE, Doney AS, Hogg S, et al. A multimodal approach to cardiovascular risk stratification in patients with type 2 diabetes incorporating retinal, genomic and clinical features. Sci Rep 2019;9:3591. [Crossref] [PubMed]
- Yoo TK, Ryu IH, Lee G, et al. Adopting machine learning to automatically identify candidate patients for corneal refractive surgery. NPJ Digit Med 2019;2:59. [Crossref] [PubMed]
- Achiron A, Gur Z, Aviv U, et al. Predicting Refractive Surgery Outcome: Machine Learning Approach With Big Data. J Refract Surg 2017;33:592-7. [Crossref] [PubMed]
- Ambrósio R Jr, Ramos I, Lopes B, et al. Assessing ectasia susceptibility prior to LASIK: the role of age and residual stromal bed (RSB) in conjunction to Belin-Ambrósio deviation index (BAD-D). Rev Bras Oftalmol 2014;73:75-80. [Crossref]
- Chan C, Saad A, Randleman JB, et al. Analysis of cases and accuracy of 3 risk scoring systems in predicting ectasia after laser in situ keratomileusis. J Cataract Refract Surg 2018;44:979-92. [Crossref] [PubMed]
- Ambrósio R Jr, Wilson S. LASIK vs LASEK vs PRK: advantages and indications. Semin Ophthalmol 2003;18:2-10. [Crossref] [PubMed]
- Awwad ST, Bteich Y, Assaf JF, et al. Prospective Objective Analysis of Corneal Haze Following Customized Transepithelial PRK Without Mitomycin C Combined With Accelerated Corneal Cross-Linking Versus Corneal Cross-Linking Alone. J Refract Surg 2024;40:e583-94. [Crossref] [PubMed]
- Yuen LH, Chan WK, Koh J, et al. A 10-year prospective audit of LASIK outcomes for myopia in 37,932 eyes at a single institution in Asia. Ophthalmology 2010;117:1236-1244.e1. [Crossref] [PubMed]
- Choi JY, Kim DE, Kim SJ, et al. Application of multimodal large language models for safety indicator calculation and contraindication prediction in laser vision correction. NPJ Digit Med 2025;8:82. [Crossref] [PubMed]
- Yoo TK, Ryu IH, Choi H, et al. Explainable Machine Learning Approach as a Tool to Understand Factors Used to Select the Refractive Surgery Technique on the Expert Level. Transl Vis Sci Technol 2020;9:8. [Crossref] [PubMed]
- Reinstein DZ, Archer TJ, Randleman JB. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg 2013;29:454-60. [Crossref] [PubMed]
- Wang Y, Ma J. Future Developments in SMILE: Higher Degree of Myopia and Hyperopia. Asia Pac J Ophthalmol (Phila) 2019;8:412-6. [Crossref] [PubMed]
- Vingopoulos F, Zisimopoulos A, Kanellopoulos AJ. Comparison of effective corneal refractive centration to the visual axis: LASIK vs SMILE, a contralateral eye digitized comparison of the postoperative result. J Cataract Refract Surg 2021;47:1511-8. [Crossref] [PubMed]
- Cui T, Wang Y, Ji S, et al. Applying Machine Learning Techniques in Nomogram Prediction and Analysis for SMILE Treatment. Am J Ophthalmol 2020;210:71-7. [Crossref] [PubMed]
- Luft N, Mohr N, Spiegel E, et al. Optimizing Refractive Outcomes of SMILE: Artificial Intelligence versus Conventional State-of-the-Art Nomograms. Curr Eye Res 2024;49:252-9. [Crossref] [PubMed]
- Tang Y, Xu J, Chen J, et al. Long-Term Destiny of Corneal Endothelial Cells in Anterior Chamber Intraocular Lens-Implanted Eyes. J Ophthalmol 2020;2020:5967509. [Crossref] [PubMed]
- Choi H, Ryu IH, Lee IS, et al. Comparison of implantation of posterior chamber phakic IOL implantation and laser vision correction in terms of corneal endothelial cells: 3-year observational paired-eye study. J Cataract Refract Surg 2023;49:936-41. [Crossref] [PubMed]
- Moshirfar M, Webster CR, Ronquillo YC. Phakic intraocular lenses: an update and review for the treatment of myopia and myopic astigmatism in the United States. Curr Opin Ophthalmol 2022;33:453-63. [Crossref] [PubMed]
- Zhang H, Gong R, Zhang X, et al. Analysis of perioperative problems related to intraocular Implantable Collamer Lens (ICL) implantation. Int Ophthalmol 2022;42:3625-41. [Crossref] [PubMed]
- Choi H, Lee SY, Lee BY, et al. Paired-eye comparison of endothelial cell density and vault height after implantable collamer lens implantation. Sci Rep 2024;14:27643. [Crossref] [PubMed]
- Choi H, Kim T, Kim SJ, et al. Predicting Postoperative Anterior Chamber Angle for Phakic Intraocular Lens Implantation Using Preoperative Anterior Segment Metrics. Transl Vis Sci Technol 2023;12:10. [Crossref] [PubMed]
- Jiang Y, Shen Y, Wang L, et al. Effect of vault on predicting postoperative refractive error for posterior chamber phakic intraocular lens based on a machine learning model. J Cataract Refract Surg 2024;50:319-27. [Crossref] [PubMed]
- Borsos B, Allaart CG, van Halteren A. Predicting stroke outcome: A case for multimodal deep learning methods with tabular and CT Perfusion data. Artif Intell Med 2024;147:102719. [Crossref] [PubMed]
- Cui C, Yang H, Wang Y, et al. Deep multimodal fusion of image and non-image data in disease diagnosis and prognosis: a review. Prog Biomed Eng (Bristol) 2023;5: [Crossref] [PubMed]
- Khosravi B, Weston AD, Nugen F, et al. Demystifying Statistics and Machine Learning in Analysis of Structured Tabular Data. J Arthroplasty 2023;38:1943-7. [Crossref] [PubMed]
- Takahashi S, Sakaguchi Y, Kouno N, et al. Comparison of Vision Transformers and Convolutional Neural Networks in Medical Image Analysis: A Systematic Review. J Med Syst 2024;48:84. [Crossref] [PubMed]
- Xie Y, Zhao L, Yang X, et al. Screening Candidates for Refractive Surgery With Corneal Tomographic-Based Deep Learning. JAMA Ophthalmol 2020;138:519-26. [Crossref] [PubMed]
- Kim J, Ryu IH, Kim JK, et al. Machine learning predicting myopic regression after corneal refractive surgery using preoperative data and fundus photography. Graefes Arch Clin Exp Ophthalmol 2022;260:3701-10. [Crossref] [PubMed]
- Kamiya K, Ryu IH, Yoo TK, et al. Prediction of Phakic Intraocular Lens Vault Using Machine Learning of Anterior Segment Optical Coherence Tomography Metrics. Am J Ophthalmol 2021;226:90-9. [Crossref] [PubMed]
- Jiang Y, Shen Y, Chen X, et al. Artificial intelligence-based refractive error prediction and EVO-implantable collamer lens power calculation for myopia correction. Eye Vis (Lond) 2023;10:22. [Crossref] [PubMed]
- Agharezaei Z, Firouzi R, Hassanzadeh S, et al. Computer-aided diagnosis of keratoconus through VAE-augmented images using deep learning. Sci Rep 2023;13:20586. [Crossref] [PubMed]
- Kim HK, Ryu IH, Choi JY, et al. A feasibility study on the adoption of a generative denoising diffusion model for the synthesis of fundus photographs using a small dataset. Discov Appl Sci 2024;6:188. [Crossref]
- Assaf JF, Yazbeck H, Reinstein DZ, et al. Enhancing the Automated Detection of Implantable Collamer Lens Vault Using Generative Adversarial Networks and Synthetic Data on Optical Coherence Tomography. J Refract Surg 2024;40:e199-207. [Crossref] [PubMed]
- Ćirković A, Katz T. Exploring the Potential of ChatGPT-4 in Predicting Refractive Surgery Categorizations: Comparative Study. JMIR Form Res 2023;7:e51798. [Crossref] [PubMed]
- Choi JY, Han E, Yoo TK. Application of ChatGPT-4 to oculomics: a cost-effective osteoporosis risk assessment to enhance management as a proof-of-principles model in 3PM. EPMA J 2024;15:659-76. [Crossref] [PubMed]
- Lopes BT, Ramos IC, Salomão MQ, et al. Enhanced Tomographic Assessment to Detect Corneal Ectasia Based on Artificial Intelligence. Am J Ophthalmol 2018;195:223-32. [Crossref] [PubMed]
- Santhiago MR, Araujo DC, Stival LR, et al. Ectasia Risk Model: A Novel Method Without Cut-off Point Based on Artificial Intelligence Improves Detection of Higher-Risk Eyes. J Refract Surg 2022;38:716-24. [Crossref] [PubMed]
- Ambrósio R Jr, Machado AP, Leão E, et al. Optimized Artificial Intelligence for Enhanced Ectasia Detection Using Scheimpflug-Based Corneal Tomography and Biomechanical Data. Am J Ophthalmol 2023;251:126-42. [Crossref] [PubMed]
- Li J, Dai Y, Mu Z, et al. Choice of refractive surgery types for myopia assisted by machine learning based on doctors' surgical selection data. BMC Med Inform Decis Mak 2024;24:41. [Crossref] [PubMed]
- Kang EM, Ryu IH, Lee G, et al. Development of a Web-Based Ensemble Machine Learning Application to Select the Optimal Size of Posterior Chamber Phakic Intraocular Lens. Transl Vis Sci Technol 2021;10:5. [Crossref] [PubMed]
- Shen Y, Wang L, Jian W, et al. Big-data and artificial-intelligence-assisted vault prediction and EVO-ICL size selection for myopia correction. Br J Ophthalmol 2023;107:201-6. [Crossref] [PubMed]
- Chen X, Ye Y, Yao H, et al. Predicting post-operative vault and optimal implantable collamer lens size using machine learning based on various ophthalmic device combinations. Biomed Eng Online 2023;22:59. [Crossref] [PubMed]
- Russo A, Filini O, Savini G, et al. Predictability of the vault after implantable collamer lens implantation using OCT and artificial intelligence in White patient eyes. J Cataract Refract Surg 2023;49:724-31. [Crossref] [PubMed]
- Chen X, Shen Y, Jiang Y, et al. Predicting Vault and Size of Posterior Chamber Phakic Intraocular Lens Using Sulcus to Sulcus-Optimized Artificial Intelligence Technology. Am J Ophthalmol 2023;255:87-97. [Crossref] [PubMed]
- Zhu J, Li FF, Li GX, et al. Enhancing Vault Prediction and ICL Sizing Through Advanced Machine Learning Models. J Refract Surg 2024;40:e126-32. [Crossref] [PubMed]
- Assaf JF, Reinstein DZ, Zakka C, et al. Deep Learning-Based Estimation of Implantable Collamer Lens Vault Using Optical Coherence Tomography. Am J Ophthalmol 2023;253:29-36. [Crossref] [PubMed]
- Shin D, Choi H, Kim D, et al. Code-Free Machine Learning Approach for EVO-ICL Vault Prediction: A Retrospective Two-Center Study. Transl Vis Sci Technol 2024;13:4. [Crossref] [PubMed]
- Di Y, Fang H, Luo Y, et al. Predicting Implantable Collamer Lens Vault Using Machine Learning Based on Various Preoperative Biometric Factors. Transl Vis Sci Technol 2024;13:8. [Crossref] [PubMed]
- Zhao H, Tang T, Lu Y, et al. Development and Validation of Data-Level Innovation Data-Balancing Machine Learning Models for Predicting Optimal Implantable Collamer Lens Size and Postoperative Vault. Ophthalmol Ther 2024;13:267-86. [Crossref] [PubMed]
- Yang Y, Long Z, Lei B, et al. Clinical decision support system based on deep learning for evaluating implantable collamer lens size and vault after implantable collamer lens surgery: a retrospective study. BMJ Open 2024;14:e081050. [Crossref] [PubMed]
- Nasser T, Hirabayashi M, Virdi G, et al. VAULT: vault accuracy using deep learning technology: new image-based artificial intelligence model for predicting implantable collamer lens postoperative vault. J Cataract Refract Surg 2024;50:448-52. [Crossref] [PubMed]
- Jin K, Ye J. Artificial intelligence and deep learning in ophthalmology: Current status and future perspectives. Adv Ophthalmol Pract Res 2022;2:100078. [Crossref] [PubMed]
- Son J, Shin JY, Kim HD, et al. Development and Validation of Deep Learning Models for Screening Multiple Abnormal Findings in Retinal Fundus Images. Ophthalmology 2020;127:85-94. [Crossref] [PubMed]
- Cen LP, Ji J, Lin JW, et al. Automatic detection of 39 fundus diseases and conditions in retinal photographs using deep neural networks. Nat Commun 2021;12:4828. [Crossref] [PubMed]
- Nguyen T, Ong J, Masalkhi M, et al. Artificial intelligence in corneal diseases: A narrative review. Cont Lens Anterior Eye 2024;47:102284. [Crossref] [PubMed]
- Yoo TK, Ryu IH, Kim JK, et al. A deep learning approach for detection of shallow anterior chamber depth based on the hidden features of fundus photographs. Comput Methods Programs Biomed 2022;219:106735. [Crossref] [PubMed]
- Peyman A, Sepahvand F, Pourazizi M, et al. Corneal biomechanics in normal and subclinical keratoconus eyes. BMC Ophthalmol 2023;23:459. [Crossref] [PubMed]
- Kanellopoulos AJ. Keratoconus Management With Customized Photorefractive Keratectomy by Artificial Intelligence Ray-Tracing Optimization Combined With Higher Fluence Corneal Crosslinking: The Ray-Tracing Athens Protocol. Cornea 2021;40:1181-7. [Crossref] [PubMed]
- Yoo TK, Choi JY, Seo JG, et al. The possibility of the combination of OCT and fundus images for improving the diagnostic accuracy of deep learning for age-related macular degeneration: a preliminary experiment. Med Biol Eng Comput 2019;57:677-87. [Crossref] [PubMed]
- Yang X, Wang Y, Liu Y, et al. Longitudinal assessment of the progression of severe keratoconus based on corneal topography. Sci Rep 2024;14:19642. [Crossref] [PubMed]
- Yoo TK, Oh E, Kim HK, et al. Deep learning-based smart speaker to confirm surgical sites for cataract surgeries: A pilot study. PLoS One 2020;15:e0231322. [Crossref] [PubMed]
- Madani A, Namazi B, Altieri MS, et al. Artificial Intelligence for Intraoperative Guidance: Using Semantic Segmentation to Identify Surgical Anatomy During Laparoscopic Cholecystectomy. Ann Surg 2022;276:363-9. [Crossref] [PubMed]
- Nespolo RG, Yi D, Cole E, et al. Feature Tracking and Segmentation in Real Time via Deep Learning in Vitreoretinal Surgery: A Platform for Artificial Intelligence-Mediated Surgical Guidance. Ophthalmol Retina 2023;7:236-42. [Crossref] [PubMed]
- Bain AP, Holcomb CN, Zeh HJ, et al. Artificial intelligence for improving intraoperative surgical care. Global Surg Educ 2024;3:73. [Crossref]
- Reinstein DZ, Archer TJ, Potter JG, et al. Refractive and Visual Outcomes of SMILE for Compound Myopic Astigmatism With the VISUMAX 800. J Refract Surg 2023;39:294-301. [Crossref] [PubMed]
- Yoo TK, Kim D, Kim JS, et al. Comparison of early visual outcomes after SMILE using VISUMAX 800 and VISUMAX 500 for myopia: a retrospective matched case-control study. Sci Rep 2024;14:11989. [Crossref] [PubMed]
- Alfonso JF, Fernández-Vega-Cueto L, Lisa C, et al. Clinical and Aberrometric Outcomes of a New Implantable Collamer Lens for Myopia and Presbyopia Correction in Phakic Patients. J Refract Surg 2023;39:589-96. [Crossref] [PubMed]
- Kanellopoulos AJ. Ray-Tracing Customization in Myopic and Myopic Astigmatism LASIK Treatments for Low and High Order Aberrations Treatment: 2-Year Visual Function and Psychometric Value Outcomes of a Consecutive Case Series. Clin Ophthalmol 2024;18:565-74. [Crossref] [PubMed]
- Zhu S, Gilbert M, Chetty I, et al. The 2021 landscape of FDA-approved artificial intelligence/machine learning-enabled medical devices: An analysis of the characteristics and intended use. Int J Med Inform 2022;165:104828. [Crossref] [PubMed]
- Cabitza F, Campagner A, Soares F, et al. The importance of being external. methodological insights for the external validation of machine learning models in medicine. Comput Methods Programs Biomed 2021;208:106288. [Crossref] [PubMed]
- Tom E, Keane PA, Blazes M, et al. Protecting Data Privacy in the Age of AI-Enabled Ophthalmology. Transl Vis Sci Technol 2020;9:36. [Crossref] [PubMed]
- Lin WC, Chen JS, Chiang MF, et al. Applications of Artificial Intelligence to Electronic Health Record Data in Ophthalmology. Transl Vis Sci Technol 2020;9:13. [Crossref] [PubMed]
- Mutasa S, Sun S, Ha R. Understanding artificial intelligence based radiology studies: What is overfitting? Clin Imaging 2020;65:96-9. [Crossref] [PubMed]
- Tjoa E, Guan C. A Survey on Explainable Artificial Intelligence (XAI): Toward Medical XAI. IEEE Trans Neural Netw Learn Syst 2021;32:4793-813. [Crossref] [PubMed]
Cite this article as: Choi JY, Choi H, Cho Y, Yoo TK. Artificial intelligence and refractive surgeries including laser vision correction and phakic IOL implantation—a narrative review. Ann Eye Sci 2025;10:7.


