
Ocular cicatricial pemphigoid: diagnosis and systemic management
Introduction
Mucous membrane pemphigoid (MMP) comprises a heterogenous group of systemic, chronic, autoimmune, and inflammatory subepithelial blistering diseases that affect mucous membranes, including the oral, conjunctival, nasal, nasopharyngeal, laryngeal, esophageal, anogenital mucosa and skin (1). Ocular involvement has been noted in about 60–70% of patients with MMP (2,3). Ocular cicatricial pemphigoid (OCP) occurs when the conjunctiva is the primary site of inflammation leading to chronic cicatrizing conjunctivitis. Linear deposition of immunoglobulin G (IgG), immunoglobulin A (IgA), immunoglobulin M (IgM), and C3 in the conjunctival basement membrane zone (BMZ) is confirmatory for OCP (4,5). Its features include conjunctival inflammation, forniceal shortening, symblepharon, ankyloblepharon, entropion, trichiasis, corneal neovascularization, opacification, and scarring.
It is a bilateral process with asymmetrical ocular involvement. It is a rare condition with a reported incidence ranging between 1 in 8,000 and 1 in 46,000 ophthalmology patients (6-9). Female predilection has been reported with a female to male ratio between 1.6:1 (4,10). OCP tends to be diagnosed in the older patient population with an average age of diagnosis between 60 and 70 years (4,11,12). However, younger adults (age <60 years) have also been observed to present with severe ocular and systemic disease, including mucocutaneous involvement (13). It is a rare condition in the pediatric population, with about 20 cases reported in the age range of 2 months to 18 years (14,15). Due to its chronic and sight-threatening nature, a diagnosis of OCP warrants prompt initiation of systemic immunosuppression to stop progression, induce prolonged and sustained remission, and avoid anatomic and functional complications. Various treatment regimens including corticosteroids, antimetabolites, alkylating, and biologic agents have been utilized to treat acute inflammation and induce remission.
Multiple review articles discussing OCP, its diagnosis and treatment modalities have been published in the past to assess emerging therapies that can be effective and serve as additional tools in the efforts to halt the progression of this sight-threatening disease. However, most reported data on current treatments are from case reports, case series, and uncontrolled clinical trials. There is a lack of randomized, controlled double-blind studies comparing the response to these agents. As a result, treatment algorithms continue to be primarily influenced by the expertise of the treating clinicians.
This review aims to thoroughly discuss the immunopathogenesis, clinical features, and diagnosis of patients with OCP. It will focus on the current immunomodulators utilized for disease management and proposed stepladder strategies. This review will discuss and provide an update on the role of combination therapy, novel use of biologics as well as the recent use of adrenocorticotropic hormone (ACTH) analog in severe recalcitrant cases. The importance of a multidisciplinary approach will be emphasized to optimize clinical care, outcomes, and quality of life of patients with OCP.
Immunopathogenesis
OCP is characterized by complex immune dysregulation and production of antibodies to components of the conjunctival BMZ. Potential target antigens in the BMZ include β-4-peptide of α-6 β-4 integrin (16-18), bullous pemphigoid antigen 2 (BP 180) (19,20), laminin 5 (21,22) and 168- and 45-kDa antigens (23,24). Rashid et al. reported specific possible epitopes in the β-4 integrin for binding of the OCP auto-antibody (25). In addition, elevated antibody titers to β-4 integrin were noted during active disease while decreased levels were associated with clinical improvement during intravenous immunoglobulin (IVIG) therapy (26).
It is hypothesized that binding of the autoantibodies at the BMZ triggers an inflammatory reaction involving the release of cytokines and recruitment of inflammatory cells. These inflammatory cells amplify and propagate the response by secreting profibrotic cytokines such as interferon-γ and transforming growth factor (TGF)-β that promote conjunctival fibrosis and scarring (27-29).
For the majority of patients, there are no identifiable precipitating factors. A combination of genetic susceptibility and environmental factors is presumed to result in the production of autoantibodies. OCP has a strong association with HLA-DQβ1*0301 (30,31). The concept of epitope spreading in the setting of severe conjunctivitis has been considered as a possible inciting factor in cases of OCP occurring after topical glaucoma drop use or after Stevens-Johnson syndrome/toxic epidermal necrolysis (1,32).
It has been proposed that loss of tolerance to epithelial basement membrane components, circulation of autoreactive T cells and production of autoantibodies (such as IgG and/or IgA) by autoreactive B cells trigger an inflammatory cascade that leads to the development of OCP (32).
OCP has been demonstrated to be associated with other autoimmune disorders, such as systemic lupus erythematosus, rheumatoid arthritis, mixed connective tissue disease, polyarteritis nodosa, and pernicious anemia (13,33).
Clinical features
Early recognition, diagnosis, and prompt therapy are pivotal in preventing functional and structural complications. In the early stages, patients may present with redness, tearing, foreign body sensation, discharge, photophobia, and decreased vision. These patients are initially diagnosed as having chronic papillary conjunctivitis unresponsive to topical therapies. Conjunctival bullae are rarely observed. The earliest indications of conjunctival scarring include subepithelial fibrosis noted in the inferior fornix and tarsal conjunctiva as well as fibrosis of the caruncle and plica (9). As OCP progresses, there is shortening of the fornices, formation of symblepharon between the palpebral and bulbar conjunctiva, and scarring of the ducts of the lacrimal and accessory lacrimal glands leading to dry ocular surface.
Eyelid abnormalities include trichiasis, distichiasis, cicatricial entropion, lagophthalmos, and ankyloblepharon. In the late stages, the patients may experience significant keratopathy, obliteration of conjunctival goblet cells, limbal stem cell deficiency, pseudo-pterygium, corneal neovascularization, opacification, thinning, and even perforation resulting in severe vision loss and blindness (10).
OCP patients are at risk for superimposed corneal infections given the compromised ocular surface and may also be at risk for glaucoma as discussed by Tauber et al. (9,34). Once a diagnosis has been made, it is important to stage the disease in order monitor progression and treatment response. Conjunctival inflammation is characterized by the degree of conjunctival injection and chemosis ranging from mild to severe states. It is also important to tease apart contributing factors such as trichiasis and ocular surface dryness that can contribute to conjunctival injection. Degree of cicatrization is demonstrated by subepithelial fibrosis, symblepharon, and forniceal shortening. Two staging systems have been defined by Foster and Mondino respectively. Foster’s staging system is outlined in Table 1 and it is based on clinical findings in the inferior fornix. Stage 2 is further subdivided based on fornix shortening and stage 3 is subdivided based on percentage of horizontal symblepharon (35,36). The various Foster stages are demonstrated in Figures 1-4.
Table 1
| Stage | Clinical features |
|---|---|
| Stage 1 | Conjunctival inflammation |
| Conjunctival subepithelial fibrosis | |
| Stage 2 | Foreshortening of inferior fornix |
| (a) 0–25% | |
| (b) 25–50% | |
| (c) 50–75% | |
| (d) 75–100% | |
| Stage 3 | Symblepharon |
| (a) 0–25% | |
| (b) 25–50% | |
| (c) 50–75% | |
| (d) 75–100% | |
| Stage 4 | Ankyloblepharon, severe ocular surface keratinization, severe sicca syndrome |
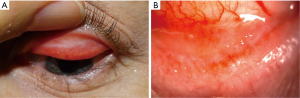


Mondino’s staging system is based on the percentage of conjunctival fornix shortening as seen in Table 2 (9,10).
Table 2
| Stage | Clinical features |
|---|---|
| Stage 1 | Loss of 0–25% of fornix depth |
| Stage 2 | Loss of 25–50% of fornix depth |
| Stage 3 | Loss of 50–75% of fornix depth |
| Stage 4 | Obliteration of fornix |
OCP is a bilateral disease, it can be asymmetrical in presentation and progression and therefore each eye should be graded individually. Patients with advanced disease are more likely to progress compared to patients with mild disease (9,10). Taking external photographs of the eyes in cardinal positions of gaze and of the inferior and superior fornices can facilitate monitoring and comparison to prior exams. The natural clinical course of OCP has been characterized by early conjunctival injection typically attributed to allergic conjunctivitis or dry eyes. It is followed by chronic conjunctival scarring and shrinkage that progresses to symblepharon, trichiasis, corneal neovascularization, and keratinization. Without treatment or in aggressive recalcitrant cases it can lead to bilateral blindness (36-38).
Complications
Dry eye disease is a frequent complication of OCP given the abnormalities in the tear film due to the disruption of the aqueous and mucin components. Structural lid abnormalities such as entropion and trichiasis can contribute to epitheliopathy, persistent epithelial defects and ulcers. Electroepilation and cryoepilation have been utilized to address trichiasis. Microbial colonization of the conjunctiva and eyelid margin can lead to blepharoconjunctivitis. Gram-positive staphylococci and gram-negative organisms have been isolated in patients with OCP (10,39). Patients should be treated with proper lid hygiene, topical antibiotic ointments and oral doxycycline to minimize the risk of infectious keratitis.
Extraocular involvement
Patients with OCP can also have extraocular manifestations with the oral mucosa being the most common site involved in the form of erosive gingivitis (9,39). Involvement of the esophagus can lead to dysphagia, reflux disease, weight loss, and fatal aspirations. Laryngeal and tracheal involvement contributes to stenosis that can manifest with hoarseness, cough, dysphonia, and breathing abnormalities. Other mucosal sites that are less frequently involved include skin, nose, genitalia, and anus (39).
Diagnosis
Diagnosing a patient with OCP is based on clinical, histological, and immunopathological findings. There are no specific laboratory tests currently available to diagnose OCP and monitor its progression and response to therapy.
Conjunctival biopsy
Conjunctival biopsies are typically obtained to confirm the diagnosis in patients with primary ocular involvement. If extraocular sites are involved that provide better access to tissue, then those sites should be biopsied first. It is important to note that performing a conjunctival biopsy may exacerbate conjunctival inflammation. Conjunctival biopsies using direct immunofluorescent or immunoperoxidase techniques facilitate the diagnosis. Linear deposition of immunoglobulins such as IgG, IgA, IgM, and C3 at the BMZ is confirmatory for OCP (39).
To increase the yield of conjunctival biopsies, it is advisable to obtain inflamed conjunctival tissue, ideally from the bulbar conjunctiva (1,39). Once obtained, it should be handled and preserved with care and processed by laboratories with experience handling conjunctival specimens to minimize the risk of inconclusive or negative results. A positive conjunctival biopsy on direct immunofluorescence (DIF) has been found to be positive in only 60–80% of cases (13,40,41). Power et al. demonstrated that using immunoperoxidase assays in immunofluorescence-negative biopsies in patients suspicious of having OCP can increase the sensitivity from 52% to 83% (12,42). It should be noted that a negative conjunctival biopsy, does not exclude the diagnosis and sometimes a repeat biopsy may be warranted. Factors such as poor tissue handling, using incorrect tissue preservation media, delayed transport to the lab and utilizing a lab with limited experience in handling conjunctival specimens can all contribute to negative results. A positive biopsy confirms the clinical findings.
Histology
Staining of conjunctival specimens with hematoxylin and eosin (H&E) reveal inflammatory infiltration with neutrophils, macrophages, T lymphocytes, eosinophils, Langerhans, dendritic, and plasma cells in the acute disease (39,43,44). During the chronic stage, there is infiltration with T-lymphocytes and macrophages. Fibrosis occurs primarily due to stimulation of fibroblasts by fibrogenic growth factors. The conjunctival epithelium in patients with OCP demonstrates squamous metaplasia with parakeratosis and keratinization, increased mast cells, and decreased goblet cell density (4,9,45). However, these findings are non-specific.
Immunologic
Patients with OCP have been found to have serum autoantibodies against α-6 β-4 integrin (18,46), α6-integrin (47), 168 kDa antigen (24), laminin 5 (22). Currently, indirect immunofluorescence (IIF) assays to detect circulating autoantibodies to components of the BMZ have played a limited role in diagnosis given the variable manifestations and circulating titer levels. Rates of antibody detection have been reported to vary in sensitivity and may be influenced by the technique and assay used, sites involved, and disease activity (27,39,43,45,48,49).
Studies on cytokine profiles have revealed elevated serum levels of IL-1α and IL-β (50), TNF-α (51), IL-5 (44), and decreased IL-6 (51) in patients with OCP. Suelves et al. demonstrated local overexpression of IL-1, IL-6, IL-12, IL-13, and IL-17 in conjunctival specimens (52).
Differential diagnosis
OCP needs to be differentiated from other diseases that can manifest with cicatrizing conjunctivitis as outlined in Table 3 (3,9,32,39). Some of these entities can be clinically indistinguishable. Diagnostic delay for patients with OCP in the UK was reported to range from 7 days to 10 years with a mean of 2.5 years (32,53).
Table 3
| Autoimmune causes |
| MMP |
| Linear IgA disease |
| Bullous pemphigoid |
| Pemphigus vulgaris |
| Stevens-Johnson syndrome/toxic epidermal necrolysis |
| Epidermolysis bullosa acquisita |
| Discoid lupus |
| Systemic lupus erythematosus |
| Sjögren’s syndrome |
| Sarcoidosis |
| Scleroderma |
| Severe atopic keratoconjunctivitis |
| Ocular rosacea |
| Graft-versus-host disease |
| Ectodermal dysplasia |
| Infectious |
| Adenovirus |
| Corynebacterium diphtheria |
| Streptococcus |
| Trachoma |
| Chronic mucocutaneous candidiasis |
| Neoplasia |
| Squamous cell carcinoma |
| Sebaceous cell carcinoma |
| Lymphoma |
| Conjunctival trauma |
| Surgical, chemical, thermal, radiation |
| Drug induced |
| Epinephrine, idoxuridine, phospholine iodide, humorsol |
| Systemic practalol |
MMP, mucous membrane pemphigoid; IgA, immunoglobulin A.
Clinical history, ocular and systemic findings, conjunctival biopsy and serologic evaluations can guide the clinician to the right diagnosis. For example, cicatrizing conjunctivitis associated with chemical injury, radiation and infectious conjunctivitis is usually not progressive. Scarring of the medial canthus is usually seen in OCP and is not commonly seen in other diseases (32).
Medical therapy
Treatment outcomes for patients with OCP prior to the use of immunomodulatory therapies (IMTs) in the 1980’s were poor and unsuccessful (32). Mondino and Brown observed progression of conjunctival scarring in 64% of patients on no treatment over a time period of 10–53 months (10). Topical and subconjunctival therapies have not been effective in controlling OCP (39,54). Systemic steroids for management of acute inflammation were used by Mondino in 1979 (55) and dapsone was first used in 1982 for ocular MMP (56). The use of immunosuppressants such as azathioprine and cyclophosphamide in patients with OCP was reported by Foster in 1980 (57). Systemic IMT is the standard of care for OCP patients and has been shown to control and slow its progression in various studies (4,10). The goals of therapy include suppressing inflammation, preventing conjunctival cicatrization, promoting healing and preventing vision loss. The various IMTs outlined in this review are used not only for OCP but also for other ocular inflammatory diseases for their steroid-sparing properties, maintaining disease control and facilitating systemic steroid tapering. Close monitoring for side effects and blood monitoring labs are recommended for all patients taking immunomodulatory agents. Once the diagnosis of OCP has been confirmed, systemic management needs to be implemented as soon as possible. It has been reported that most conjunctival scarring occurs during episodes of active inflammation (55). Many patients are diagnosed at advanced stages (32) and the time interval between the start of therapy and total inflammation control can play a major role in disease progression (58). Even despite systemic IMT, progression of cicatrization has been reported in 10–53% of OCP patients (54,59) and some patients still progress to blindness (12,37,60,61). The progression appears to occur at a faster pace in patients who are younger (13). Therapy is offered to patients with active and progressive disease (39). Those patients who have end-stage disease or “burned out disease” may not benefit from IMT since no current therapy is available to revert the cicatricial changes on the ocular surface (32,39).
It should be note that about 25% of patients with OCP may not require IMT because of limited symptoms and scarring, mild or no inflammation and slow or no progression (32).
No large-scale randomized controlled trials have been done to compare therapies in patients with OCP and establishing a standard treatment protocol is challenging. The clinician must consider the patient’s age, past medical history, site involvement, staging, degree of progression, and potential toxicity to determine the best treatment plan. Cost of therapy is another factor to consider since insurance coverage will determine the cost to the patient with many of these agents needing prior authorization. Anti-metabolite therapies such as methotrexate, mycophenolate, and azathioprine tend to be more affordable given their long-standing and generic availability compared to more recent biologic therapies such as adalimumab, rituximab, and infliximab that tend to be more expensive.
Combination therapy in a stepladder approach based on clinical severity is usually needed to achieve inflammation control and remission. For mild to moderate OCP, dapsone can be used as the initial therapy. If the patient experiences intolerable side effects, then an anti-metabolite such as methotrexate, mycophenolate mofetil (MMF), and azathioprine can be added or substituted. Cyclophosphamide with corticosteroids and rituximab with or without IVIG is typically reserved for severe or recalcitrant cases.
Sulfonamide antibiotics
Diaminodiphenylsulfone
Dapsone is a synthetic sulfone with anti-inflammatory and anti-microbial properties. It is a competitive antagonist of par-aminobenzoic acid (PABA) affecting bacterial use of PABA for folic acid synthesis. It also affects neutrophil chemotaxis and phagocytosis (39,62). It can be used for mild OCP. Initial dosing starts at 25 mg twice daily (BID) and can be titrated up to 50 mg BID, with the highest dose at 150 mg daily. If there is no response noted after 3 months, then therapy should be switched to an anti-metabolite agent such as methotrexate. Dapsone should be avoided in patients with sulfa allergy, glucose-6-phosphate dehydrogenase (G6PD) deficiency, hemoglobin M deficiency, and methemoglobin reductase deficiency. Reported side effects include malaise, skin rashes, nausea and abdominal pain, agranulocytosis, hemolytic anemia, hepatitis, and peripheral neuropathy pain. Dapsone has been noted to have the highest number of complications leading to its discontinuation in various studies (54,63). Patients should have baseline complete blood count (CBC), liver function tests, and G6PD activity levels and methemoglobin status (9,39).
Sulfasalazine and sulphapyridine
Sulfasalazine has been reported to inhibit proliferation of T-lymphocytes, decrease production of immunoglobulins and cytokines and hinder neutrophil chemotaxis. Doan et al. reported the use of sulfasalazine in patients experiencing adverse effects from Dapsone therapy (63). The dosing can range from 1 to 4 g per day. Hemolytic anemia occurs less frequently when compared to dapsone. Other possible side effects include headache, dizziness, and gastrointestinal changes (64). Elder et al. reported sulphapyridine to be clinically effective in 50% of patients with moderate inflammation and had few side effects. It can be a good alternative to dapsone (38).
Methotrexate
Methotrexate inhibits DNA replication, reduces cellular and humoral responses, induces T-cell apoptosis, alters B-cell response, and inhibits cytokine production (65). It has been used extensively for ocular inflammatory conditions. It can be an alternative if a patient has an incomplete response to Dapsone or is unable to tolerate it. It can be taken orally or subcutaneously on a weekly basis. Dosing can start 7.5 mg with subsequent titration up to 25 mg based on clinical response. Its weekly dosing can facilitate patient compliance and facilitate its use as first-line therapy compared to other agents such as mycophenolate or azathioprine that need to be taken on a daily basis.
Reported side effects include fatigue, nausea, vomiting, hepatoxicity, hair thinning, and pulmonary fibrosis. Patients should take folic acid to minimize gastrointestinal symptoms and ulcers. Expected onset of action ranges from 3 to 6 weeks (66). Methotrexate should be avoided in patients with known liver disease and/or alcohol abuse. Since it is teratogenic, it should be used with caution in patients of childbearing age. Liver function tests and complete blood cell counts should be done as part of monitoring. McCluskey et al. showed that methotrexate was effective in controlling inflammation in 89% of mild to moderate OCP cases and highlighted that methotrexate monotherapy can be efficacious and well tolerated as first-line treatment (67). In comparison, Shi et al. conducted a retrospective case series, where low-dose methotrexate was used in patients with advanced disease with four patients at Foster stage 3 and seven patients at stage 4. Conjunctival inflammation was noted to improve in five patients with improvement in ocular surface keratinization, however, three patients noted no improvement (68).
MMF
MMF is the ester prodrug of mycophenolate acid. It inhibits purine synthesis, DNA synthesis, B and T cell proliferation, cell adhesion, lymphocytic chemotaxis and reduces antibody synthesis (65). The starting dose is 500 mg daily to twice a day by oral route on an empty stomach. The dose can be increased to 1 gram twice a day with a maximum dose of 3 grams a day (39). The most common side effects are gastrointestinal irritability, elevated liver function tests, and bone marrows suppression. It is considered teratogenic. It can be used in patients with an inadequate response to methotrexate or if they have experienced intolerance. Its onset of action can range from 2 weeks to 3 months (66). Saw et al. observed that about 81% of patients treated with MMF had response to treatment and only 15% of patients developed side effects. It was noted to be effective and well tolerated for moderately active OCP (61). A retrospective study by Doycheva et al. included 19 eyes of 10 patients with OCP treated with MMF. They demonstrated control of inflammation in 58% of eyes, but noted mild progression of cicatrization in 42% of eyes (69). MMF was noted to have a better side effect profile, lower rate of discontinuation and can also be considered as first-line therapy. Blood monitoring with complete blood cell count, liver function tests, and basic metabolic panel is also indicated.
Azathioprine
Azathioprine inhibits DNA and RNA synthesis, T lymphocyte function, antibody production (39,65). Dave and Vickers observed success in inflammation control with azathioprine in patients with mucus membrane pemphigoid (70). Its use for OCP has been documented in various retrospective studies and case reports (37,61,71). Saw et al. showed that azathioprine was able to control inflammation in only 48% of 37 treatment episodes in patients with biopsy-proven OCP that had not responded to a sulfonamide. Its discontinuation rate due to side effects was higher when compared to other therapies (61). It has been noted to be moderately effective as monotherapy for noninfectious ocular inflammation (72). The recommended starting dose is 2.0 mg/kg/day orally with a maximum dose of 3 mg/kg/day. It should be avoided in patients with low or absent thiopurine methyltransferase (TPMT) enzyme activity. Response to therapy can occur from 1 to 3 months. Common reported side effects are arthralgias, headache, malaise, leukopenia, bone marrow suppression, and liver function abnormalities. Follow-up liver function tests and CBC should be repeated every 6 weeks.
Severe or refractory disease
For patients with severe or refractory OCP disease, unresponsive to conventional therapies aggressive therapy needs to be implemented. Combination of cyclophosphamide with prednisone is highly successful in controlling inflammation and achieving ocular remission (1,11,12,73). Recent studies have demonstrated the effectiveness of using IVIG and rituximab as monotherapy or combination treatment (74-77). Systemic glucocorticoids, either in oral or intravenous form, can have a useful role in treating acute inflammation while the immunosuppressants take effect. However, corticosteroids should not be used as monotherapy for long-term management due to unfavorable side effect profile and high risk of disease recurrence after tapering and discontinuation (4,10). Hardy et al. observed risk for pneumonia leading to death, gastrointestinal bleeding, and cerebrovascular events in OCP patients treated with high-dose corticosteroids (2).
Cyclophosphamide
Cyclophosphamide is an alkylating agent that has a cytotoxic effect on proliferating cells such as lymphocytes and its use has been shown to achieve inflammation quiescence in patients with OCP (78). Thorne et al. also demonstrated treatment with cyclophosphamide and prednisone was strongly associated in achieving remission (11). Oral dosing starts at 1–2 mg/kg per day. Intravenous dosing starts at 1 g/m2 infused every 2 weeks. The dose is titrated to maintain the peripheral white blood cell count above 3,000 cells/mm3, absolute neutrophil count above 1,000/mm3, and platelet count above 70,000 platelets/mL (27,39). Friedman et al. reported low dosed pulsed treatments (500 mg given monthly) appear to be better tolerated by elderly OCP patients (79,80). Adjunctive oral corticosteroids can be dosed orally at 1–2 mg/kg/day or intravenously with methylprednisolone from 500 mg to 1 gram while the response to cyclophosphamide takes effect in 6 to 8 weeks. Once a response has been observed, then oral prednisone is tapered over a 3-month period. Given the risk of toxicity and long-term side effects with cyclophosphamide, patients are treated for 12 months and are then switched to other immunosuppressants. Adverse reactions include leukopenia, thrombocytopenia, infections, hemorrhagic cystitis, gastrointestinal distress, renal toxicity, hair loss, amenorrhea, premature ovarian failure, and azoospermia. Saw et al. noted that adverse events altered therapy in about 31% of patients, including two life-threatening episodes of pancytopenia and hepatotoxicity (61). Due to its teratogenic properties, cyclophosphamide is contraindicated in pregnancy. Close monitoring of CBC and urinalysis should be done every 2 weeks. Bladder toxicity is minimized by using intravenous therapy.
IVIG
IVIG is employed in patients with active OCP who have recalcitrant disease, are unresponsive to other immunosuppressants or have intolerable side effects (74,76,77). The mechanism of action for IVIG is complex, but it is known to have anti-inflammatory effects by interfering with B cell function by blocking the Fc portion of the receptor (81), reducing pro-inflammatory cytokines such as IL-1 (82), TNF-α (83), stimulating production of anti-inflammatory cytokines including IL-6 (84), IL-1Ra (85) and inactivating complement (86). Letko et al. reported a decrease in the antibody titer to human β4-integrin in OCP patients treated with IVIG correlating with decreased inflammation (26). In 2004, his group showed that IVIG is effective in stopping OCP progression and inducing prolonged and sustained remission in eight patients while progression was noted in four patients treated with conventional immunosuppressants. They also demonstrated that IVIG was safer and better tolerated compared to other immunomodulators (74). It is dosed at 2–3 g/kg/cycle infused over 4- to 5-hour infusions for 3 consecutive days every month. The number of cycles is dependent on the patient’s clinical state. Antibody titers are obtained prior starting IVIG and every month while receiving therapy. It is overall very well tolerated; however, possible adverse effects include fatigue, headaches, anemia, volume overload, and vaso-occlusive events. IVIG should be avoided in patients with IgA deficiency or those with a prior reaction to intramuscular or intravenous human immunoglobulin (39).
Rituximab
Rituximab is a chimeric monoclonal antibody directed at the CD20 surface antigen on pre-B and mature B lymphocytes (87). It was initially used in patients with B-cell lymphomas and eventually used for rheumatoid arthritis, systemic lupus erythematosus, granulomatosis with polyangiitis, microscopy polyangiitis, and pemphigus vulgaris (88). Variable responses and effective use of rituximab in MMP and OCP have been documented in various reports (75,87,89-98). It has been used as monotherapy or in combination with other agents. The dosing has ranged from the rheumatoid arthritis protocol (1,000 mg at days 0 and 14), the lymphoma protocol (375 mg/kg weekly for month), and Foster protocol (375 mg/m2 given weekly for 8 weeks, then monthly for 4 months). Bevans et al. showed that rituximab can be used as an adjunctive and rescue therapy to induce remission when used in combination with other IMT agents in resistant cases of OCP. It also recommended its use earlier in the disease course to stop progression and preserve vision (99). In a study by Le Roux-Villet et al., rituximab treatment led to resolution of conjunctival inflammation in nine of the 10 patients with severe OCP with ocular remission attained within a median of 10 weeks. It was also recommended that it could be used as monotherapy rather than combined with other IMT (93). Most commonly reported adverse reactions include nausea, infusion reactions, infection, anemia, leukopenia, hypogammaglobulinemia, liver function abnormalities, multifocal leukoencephalopathy (75,99,100). Onset of action can occur in about 12 weeks (66).
Combination therapy of rituximab and IVIG was studied in a retrospective study in 12 patients with recalcitrant OCP. Six patients were treated with rituximab and IVIG while the 6 control patients with comparable OCP severity were treated with other systemic IMT including methotrexate, MMF, azathioprine, dapsone, oral prednisone. The patients in the study group were treated with a uniform protocol of rituximab (375 mg/m2 given weekly for 8 weeks, then monthly for 4 months) and IVIG (2 g/kg per cycle divided into three equal parts and infused over 3 consecutive days, at monthly intervals until B-cell levels normalized, then the intervals were increased to 6, 8, 10, 12, 14, 16 weeks. Disease progression was halted and total blindness was prevented in the six patients in the rituximab and IVIG group while all six patients in the control group had progression and became blind in both eyes. The study highlighted the importance of checking pre-treatment and post-treatment B-cell levels. It also outlined the criteria for patients who may benefit from this treatment such as those experiencing complete failure on other therapies, intolerable side effects, continuous progression, poor response to IVIG monotherapy and severe threat of blindness (75).
Tumor necrosis factor-α inhibitors
Studies have shown elevated TNF-α levels in the serum of patients with MMP when compared to controls (51,101). TNF-α has been shown to play a role in early inflammation and scarring (102). As a result, TNF-α inhibitors have been studied to assess their impact in treating OCP.
Etarnecept is a recombinant human dimeric fusion protein that acts as a competitive inhibitor of TNF-α by binding to soluble or receptor bound molecules of TNF-α. Case reports have shown the use of etarnecept in patients non-responsive to first-line therapies (103-106). Infliximab is a chimeric IgG monoclonal antibody directed at soluble and transmembrane forms of TNF-α. It was utilized by Heffernan et al. in a patient with severe MMP who had not responded to dapsone, prednisone and cyclophosphamide and IVIG (107). It was dosed at 5 mg/kg and infused at 8-week intervals. Infliximab onset of action can occur in 1 to 2 weeks (66). The patient was noted to have rapid improvement (107).
ACTH analogues
ACTH is a melanocortin produced by the hypothalamic-pituitary axis and promotes the production of steroid hormones, such as cortisol (108). Repository corticotropin injection (RCI) has been approved for the treatment of severe autoimmune and allergic conditions. It has also been employed in treating chronic, progressive and refractory ocular inflammation (109). In a case report, a 75-year-old female with biopsy positive OCP refractory and intolerant to other IMT was treated with subcutaneous ACTH. The patient received subcutaneous ACTH gel injections twice weekly after failing other IMT. Significant improvement in conjunctival inflammation was observed while the systemic steroids were tapered and the patient tolerated treatment with no significant side effects (108). Sharon et al. conducted a retrospective study with 15 patients with active OCP with treatment failure with other immunomodulators and observed RCIs can be an alternative treatment in patients with refractive and severe OCP (109).
Table 4 summarizes the treatment protocol outlined by Ahmed et al. based on disease activity and staging (39).
Table 4
| Disease activity | Foster stage | Therapy |
|---|---|---|
| Mild | Stages 1–3 | Dapsone → methotrexate → MMF or azathioprine |
| Severe | Stage 4 → recalcitrant | Cyclophosphamide (PO or IV) & corticosteroids (PO or IV) → IVIG ± rituximab |
Recent therapies with potential benefits: TNF-α inhibitors, ACTH. OCP, ocular cicatricial pemphigoid; MMF, mycophenolate mofetil; PO, oral; IV, intravenous; IVIG, intravenous immunoglobulin; ACTH, adrenocorticotropic hormone.
The systematic approach outlined indicates that for mild disease defined as Foster stage 1 to 3, initial therapy options include dapsone, however, methotrexate can be an alternative if a patient has an incomplete response or is unable to tolerate it. If methotrexate is not a viable option, then mycophenolate or azathioprine can be alternatives. The arrows indicate the possible next step in management. For severe disease, defined as stage 4, then combination therapy can be an option using cyclophosphamide with corticosteroids or rituximab with IVIG. Newer agents such as TNF-α inhibitors and ACTH analog can have a role for recalcitrant cases.
Local ocular considerations and management
In addition to ensuring effective systemic control, patients should be closely monitored for ocular surface dryness, infection risk and trichiasis. Dry eye disease should be aggressively treated with preservative-free lubricants, topical drops, punctal plugs or punctal cautery. Corneal punctate epitheliopathy, persistent epithelial defects, and corneal perforations can present challenges and lead to corneal decompensation and vision loss. Lid hygiene is encouraged given the increased risk of blepharoconjunctivitis. Trichiasis should be addressed with epilation. Electrolysis and cryoepilation offer more long-lasting results compared to manual epilation (39). Cryoepilation should be performed when OCP is quiescent to avoid triggering an exacerbation.
Prognosis
Once complete control of inflammation is achieved, therapy should be continued for at least 12 months. If quiescence has been achieved, then therapy should be gradually tapered while observing closely for recurrences. Patients should be counseled about the importance of life-long follow-up since one-third of patients can relapse (4,37). If the disease is severe and has led to blindness in one eye, then life-long therapy should be discussed with patient. It should be noted that about 6% to 10% of patients may continue to have inflammation progression with irreversible complications despite being on therapy (9,12,37,58). One-third of patients treated with systemic IMT can experience prolonged periods of remission. However, Neumann et al. showed that relapses can occur in 22% of patients in remission off systemic treatment and needed reinstitution of treatment (58). OCP patients benefit from a multidisciplinary approach involving primary care providers, rheumatologists, gastroenterologists, ophthalmologists, dermatologists, gynecologists, otolaryngologists, dentists, oral surgeons based on their specific mucous membrane involvement. Stratifying patients based on high-risk features such as ocular, esophageal, laryngeal, nasopharyngeal, genital involvement, and rapid progression can help in escalating therapy as appropriate and ensure close follow-up (1,107).
Conclusions
OCP is a chronic autoimmune sight-threatening condition primarily affecting an elderly patient population. High level of suspicion for OCP is needed for prompt diagnosis and initiation of therapy. Due to its systemic and progressive nature and risk of vision and life-threatening complications, systemic immunosuppression is warranted in most cases. Various immunomodulatory agents as outlined in this review have been utilized to control inflammation, arrest progression, induce remission and preserve vision. Current treatment protocols may vary given the rare nature of this disease, small sample sizes and uncontrolled nature of published studies. Even despite immunosuppressive therapy and control of conjunctival inflammation, cicatrization may still progress. OCP presents a diagnostic and therapeutic challenge to clinicians while causing a significant impact in patients’ well-being and quality of life. Patients benefit from a multidisciplinary approach to address disease manifestation and complications. They need to be counseled about the need for close observation while on IMT and life-long monitoring to assess OCP activity.
Acknowledgments
The author would like to thank Dr. Ninani Kombo for the photo courtesy of Figures 1B,2A,3A,4.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Joann Kang and Roy S. Chuck) for the series “Ocular Surface Reconstruction/Transplantation” published in Annals of Eye Science. The article has undergone external peer review.
Peer Review File: Available at https://aes.amegroups.com/article/view/10.21037/aes-22-41/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-22-41/coif). The series “Ocular Surface Reconstruction/Transplantation” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 2002;138:370-9. [Crossref] [PubMed]
- Hardy KM, Perry HO, Pingree GC, et al. Benign mucous membrane pemphigoid. Arch Dermatol 1971;104:467-75. [Crossref] [PubMed]
- Saw VP, Dart JK. Ocular mucous membrane pemphigoid: diagnosis and management strategies. Ocul Surf 2008;6:128-42. [Crossref] [PubMed]
- Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc 1986;84:527-663. [PubMed]
- Mondino BJ. Cicatricial pemphigoid and erythema multiforme. Ophthalmology 1990;97:939-52. [Crossref] [PubMed]
- Bedell AJ. Ocular pemphigus: a clinical presentation of kodachromes. Trans Am Ophthalmol Soc 1964;62:109-22. [PubMed]
- Smith RC, Myers EA, Lamb HD. Ocular and oral pemphigus: report of a case with anatomic findings in the eyeball. Arch Ophthal 1934;11:635-40. [Crossref]
- Bettelheim H, Kraft D, Zehetbauer G. On the so-called ocular pemphigus (pemphigus ocularis, pemphigus conjunctivae). Klin Monbl Augenheilkd 1972;160:65-75. [PubMed]
- Foster CS, Azar DZ, Dohlman CH. Smolin and Thoft's. The cornea: scientific foundations and clinical practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Mondino BJ, Brown SI. Ocular cicatricial pemphigoid. Ophthalmology 1981;88:95-100. [Crossref] [PubMed]
- Thorne JE, Woreta FA, Jabs DA, et al. Treatment of ocular mucous membrane pemphigoid with immunosuppressive drug therapy. Ophthalmology 2008;115:2146-2152.e1. [Crossref] [PubMed]
- Tauber J, Sainz de la Maza M, Foster CS. Systemic chemotherapy for ocular cicatricial pemphigoid. Cornea 1991;10:185-95. [Crossref] [PubMed]
- Rauz S, Maddison PG, Dart JK. Evaluation of mucous membrane pemphigoid with ocular involvement in young patients. Ophthalmology 2005;112:1268-74. [Crossref] [PubMed]
- Lavallée A, Bourret-Massicotte D, Laughrea PA. Childhood Mucous Membrane Pemphigoid: A Case With Exclusively Ocular Involvement. Cornea 2013;32:1399-401. [Crossref] [PubMed]
- Flores-Climente V, Rozas-Muñoz E, Martínez-Grau A, et al. Childhood ocular mucous membrane pemphigoid successfully treated with rituximab. Pediatr Dermatol 2019;36:984-5. [Crossref] [PubMed]
- Chan RY, Bhol K, Tesavibul N, et al. The role of antibody to human beta4 integrin in conjunctival basement membrane separation: possible in vitro model for ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci 1999;40:2283-90. [PubMed]
- Kumari S, Bhol KC, Simmons RK, et al. Identification of ocular cicatricial pemphigoid antibody binding site(s) in human beta4 integrin. Invest Ophthalmol Vis Sci 2001;42:379-85. [PubMed]
- Tyagi S, Bhol K, Natarajan K, et al. Ocular cicatricial pemphigoid antigen: partial sequence and biochemical characterization. Proc Natl Acad Sci U S A 1996;93:14714-9. [Crossref] [PubMed]
- Bédane C, McMillan JR, Balding SD, et al. Bullous pemphigoid and cicatricial pemphigoid autoantibodies react with ultrastructurally separable epitopes on the BP180 ectodomain: evidence that BP180 spans the lamina lucida. J Invest Dermatol 1997;108:901-7. [Crossref] [PubMed]
- Balding SD, Prost C, Diaz LA, et al. Cicatricial pemphigoid autoantibodies react with multiple sites on the BP180 extracellular domain. J Invest Dermatol 1996;106:141-6. [Crossref] [PubMed]
- Kirtschig G, Marinkovich MP, Burgeson RE, et al. Anti-basement membrane autoantibodies in patients with anti-epiligrin cicatricial pemphigoid bind the alpha subunit of laminin 5. J Invest Dermatol 1995;105:543-8. [Crossref] [PubMed]
- Fujimoto W, Toi Y, Okazaki F, et al. Anti-epiligrin cicatricial pemphigoid with IgG autoantibodies to the beta and gamma subunits of laminin 5. J Am Acad Dermatol 1999;40:637-9. [Crossref] [PubMed]
- Smith EP, Taylor TB, Meyer LJ, et al. Identification of a basement membrane zone antigen reactive with circulating IgA antibody in ocular cicatricial pemphigoid. J Invest Dermatol 1993;101:619-23. [Crossref] [PubMed]
- Ghohestani RF, Nicolas JF, Rousselle P, et al. Identification of a 168-kDa mucosal antigen in a subset of patients with cicatricial pemphigoid. J Invest Dermatol 1996;107:136-9. [Crossref] [PubMed]
- Rashid KA, Foster CS, Ahmed AR. Identification of epitopes within integrin β4 for binding of auto-antibodies in ocular cicatricial and mucous membrane pemphigoid: preliminary report. Invest Ophthalmol Vis Sci 2013;54:7707-16. [Crossref] [PubMed]
- Letko E, Bhol K, Foster SC, et al. Influence of intravenous immunoglobulin therapy on serum levels of anti-beta 4 antibodies in ocular cicatricial pemphigoid. A correlation with disease activity. A preliminary study. Curr Eye Res 2000;21:646-54. [Crossref] [PubMed]
- Ocular Cicatricial Pemphigoid. In: Zone JJ. editor. UpToDate. Accessed on June 7, 2022. Available online: https://www.uptodate.com/contents/search?search=ocular%20cicatricial%20pemphigoid&sp=0&searchType=PLAIN_TEXT&source=USER_INPUT&searchControl=TOP_PULLDOWN&autoComplete=true
- Elder MJ, Dart JK, Lightman S. Conjunctival fibrosis in ocular cicatricial pemphigoid--the role of cytokines. Exp Eye Res 1997;65:165-76. [Crossref] [PubMed]
- Razzaque MS, Foster CS, Ahmed AR. Role of connective tissue growth factor in the pathogenesis of conjunctival scarring in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci 2003;44:1998-2003. [Crossref] [PubMed]
- Yunis JJ, Mobini N, Yunis EJ, et al. Common major histocompatibility complex class II markers in clinical variants of cicatricial pemphigoid. Proc Natl Acad Sci U S A 1994;91:7747-51. [Crossref] [PubMed]
- Zakka LR, Reche P, Ahmed AR. Role of MHC Class II genes in the pathogenesis of pemphigoid. Autoimmun Rev 2011;11:40-7. [Crossref] [PubMed]
- Dart JK. The 2016 Bowman Lecture Conjunctival curses: scarring conjunctivitis 30 years on. Eye (Lond) 2017;31:301-32. [Crossref] [PubMed]
- Anzaar F, Cabrita F, Ahmed M, et al. The frequency of other autoimmune disorders in patients with ocular cicatricial pemphigoid. Acta Ophthalmol 2012;90:e253-4. [Crossref] [PubMed]
- Tauber J, Melamed S, Foster CS. Glaucoma in patients with ocular cicatricial pemphigoid. Ophthalmology 1989;96:33-7. [Crossref] [PubMed]
- Ahmed AR, Foster S, Zaltas M, et al. Association of DQw7 (DQB1*0301) with ocular cicatricial pemphigoid. Proc Natl Acad Sci U S A 1991;88:11579-82. [Crossref] [PubMed]
- Tauber J, Jabbur N, Foster CS. Improved detection of disease progression in ocular cicatricial pemphigoid. Cornea 1992;11:446-51. [Crossref] [PubMed]
- Foster CS, Neumann R, Tauber J. Long-term results of systemic chemotherapy for ocular cicatricial pemphigoid. Doc Ophthalmol 1992;82:223-9. [Crossref] [PubMed]
- Elder MJ, Bernauer W, Leonard J, et al. Progression of disease in ocular cicatricial pemphigoid. Br J Ophthalmol 1996;80:292-6. [Crossref] [PubMed]
- Ahmed M, Zein G, Khawaja F, et al. Ocular cicatricial pemphigoid: pathogenesis, diagnosis and treatment. Prog Retin Eye Res 2004;23:579-92. [Crossref] [PubMed]
- Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology 2004;111:45-52. [Crossref] [PubMed]
- Bernauer W, Elder MJ, Leonard JN, et al. The value of biopsies in the evaluation of chronic progressive conjunctival cicatrisation. Graefes Arch Clin Exp Ophthalmol 1994;232:533-7. [Crossref] [PubMed]
- Power WJ, Neves RA, Rodriguez A, et al. Increasing the diagnostic yield of conjunctival biopsy in patients with suspected ocular cicatricial pemphigoid. Ophthalmology 1995;102:1158-63. [Crossref] [PubMed]
- Elder MJ, Lightman S. The immunological features and pathophysiology of ocular cicatricial pemphigoid. Eye (Lond) 1994;8:196-9. [Crossref] [PubMed]
- Letko E, Bhol K, Colon J, et al. Biology of interleukin-5 in ocular cicatricial pemphigoid. Graefes Arch Clin Exp Ophthalmol 2002;240:565-9. [Crossref] [PubMed]
- Kinoshita S, Kiorpes TC, Friend J, et al. Goblet cell density in ocular surface disease. A better indicator than tear mucin. Arch Ophthalmol 1983;101:1284-7. [Crossref] [PubMed]
- Bhol KC, Dans MJ, Simmons RK, et al. The autoantibodies to alpha 6 beta 4 integrin of patients affected by ocular cicatricial pemphigoid recognize predominantly epitopes within the large cytoplasmic domain of human beta 4. J Immunol 2000;165:2824-9. [Crossref] [PubMed]
- Kanitakis J, Zambruno G, Vassileva S, et al. Alpha-6 (CD 49f) integrin expression in genetic and acquired bullous skin diseases. A comparison of its distribution with bullous pemphigoid antigen. J Cutan Pathol 1992;19:376-84. [Crossref] [PubMed]
- Ahmed AR, Khan KN, Wells P, et al. Preliminary serological studies comparing immunofluorescence assay with radioimmunoassay. Curr Eye Res 1989;8:1011-9. [Crossref] [PubMed]
- Franklin RM, Fitzmorris CT. Antibodies against conjunctival basement membrane zone. Occurrence in cicatricial pemphigoid. Arch Ophthalmol 1983;101:1611-3. [Crossref] [PubMed]
- Kumari S, Bhol KC, Rehman F, et al. Interleukin 1 components in cicatricial pemphigoid. Role in intravenous immunoglobulin therapy. Cytokine 2001;14:218-24. [Crossref] [PubMed]
- Lee SJ, Li Z, Sherman B, et al. Serum levels of tumor necrosis factor-alpha and interleukin-6 in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci 1993;34:3522-5. [PubMed]
- Suelves AM, Zhao TZ, Siddique SS, et al. Profile of local interleukin expression in a cohort of ocular cicatricial pemphigoid patients. Invest Ophthalmol Vis Sci 2012;53:8112-7. [Crossref] [PubMed]
- Radford CF, Rauz S, Williams GP, et al. Incidence, presenting features, and diagnosis of cicatrising conjunctivitis in the United Kingdom. Eye (Lond) 2012;26:1199-208. [Crossref] [PubMed]
- Miserocchi E, Baltatzis S, Roque MR, et al. The effect of treatment and its related side effects in patients with severe ocular cicatricial pemphigoid. Ophthalmology 2002;109:111-8. [Crossref] [PubMed]
- Mondino BJ, Brown SI, Lempert S, et al. The acute manifestations of ocular cicatricial pemphigoid: diagnosis and treatment. Ophthalmology 1979;86:543-55. [Crossref] [PubMed]
- Rogers RS 3rd, Seehafer JR, Perry HO. Treatment of cicatricial (benign mucous membrane) pemphigoid with dapsone. J Am Acad Dermatol 1982;6:215-23. [Crossref] [PubMed]
- Foster CS. Immunosuppressive therapy for external ocular inflammatory disease. Ophthalmology 1980;87:140-50. [Crossref] [PubMed]
- Neumann R, Tauber J, Foster CS. Remission and recurrence after withdrawal of therapy for ocular cicatricial pemphigoid. Ophthalmology 1991;98:858-62. [Crossref] [PubMed]
- Mondino BJ, Brown SI. Immunosuppressive therapy in ocular cicatricial pemphigoid. Am J Ophthalmol 1983;96:453-9. [Crossref] [PubMed]
- Elder MJ, Lightman S, Dart JK. Role of cyclophosphamide and high dose steroid in ocular cicatricial pemphigoid. Br J Ophthalmol 1995;79:264-6. [Crossref] [PubMed]
- Saw VP, Dart JK, Rauz S, et al. Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes. Ophthalmology 2008;115:253-261.e1. [Crossref] [PubMed]
- Dapsone. In: Zone JJ. editor. UpToDate. Accessed on June 7, 2022. Available online: https://www.uptodate.com/contents/dapsone-systemic-drug-information?search=dapsone&source=panel_search_result&selectedTitle=1%7E150&usage_type=panel&showDrugLabel=true&display_rank=1
- Doan S, Lerouic JF, Robin H, et al. Treatment of ocular cicatricial pemphigoid with sulfasalazine. Ophthalmology 2001;108:1565-8. [Crossref] [PubMed]
- Sobolewska B, Deuter C, Zierhut M. Current medical treatment of ocular mucous membrane pemphigoid. Ocul Surf 2013;11:259-66. [Crossref] [PubMed]
- Castiblanco C, Foster CS. Review of Systemic Immunosuppression for Autoimmune Uveitis. Ophthalmol Ther 2014;3:17-36. [Crossref] [PubMed]
- Foster CS, Kothari S, Anesi SD, et al. The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv Ophthalmol 2016;61:1-17. [Crossref] [PubMed]
- McCluskey P, Chang JH, Singh R, et al. Methotrexate therapy for ocular cicatricial pemphigoid. Ophthalmology 2004;111:796-801. [Crossref] [PubMed]
- Shi Y, Xie C, He Y, et al. Efficacy and adverse reactions of methotrexate in the treatment of ocular cicatricial pemphigoid: A case series study. Medicine (Baltimore) 2018;97:e12338. [Crossref] [PubMed]
- Doycheva D, Deuter C, Blumenstock G, et al. Long-term results of therapy with mycophenolate mofetil in ocular mucous membrane pemphigoid. Ocul Immunol Inflamm 2011;19:431-8. [Crossref] [PubMed]
- Dave VK, Vickers CF. Azathioprine in the treatment of muco-cutaneous pemphigoid. Br J Dermatol 1974;90:183-6. [Crossref] [PubMed]
- Lugović L, Buljan M, Situm M, et al. Unrecognized cicatricial pemphigoid with oral manifestations and ocular complications. A case report. Acta Dermatovenerol Croat 2007;15:236-42. [PubMed]
- Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol 2009;148:500-509.e2. [Crossref] [PubMed]
- Foster CS, Wilson LA, Ekins MB. Immunosuppressive therapy for progressive ocular cicatricial pemphigoid. Ophthalmology 1982;89:340-53. [Crossref] [PubMed]
- Letko E, Miserocchi E, Daoud YJ, et al. A nonrandomized comparison of the clinical outcome of ocular involvement in patients with mucous membrane (cicatricial) pemphigoid between conventional immunosuppressive and intravenous immunoglobulin therapies. Clin Immunol 2004;111:303-10. [Crossref] [PubMed]
- Foster CS, Chang PY, Ahmed AR. Combination of rituximab and intravenous immunoglobulin for recalcitrant ocular cicatricial pemphigoid: a preliminary report. Ophthalmology 2010;117:861-9. [Crossref] [PubMed]
- Foster CS, Ahmed AR. Intravenous immunoglobulin therapy for ocular cicatricial pemphigoid: a preliminary study. Ophthalmology 1999;106:2136-43. [Crossref] [PubMed]
- Sami N, Letko E, Androudi S, et al. Intravenous immunoglobulin therapy in patients with ocular-cicatricial pemphigoid: a long-term follow-up. Ophthalmology 2004;111:1380-2. [Crossref] [PubMed]
- Durrani K, Papaliodis GN, Foster CS. Pulse IV cyclophosphamide in ocular inflammatory disease: efficacy and short-term safety. Ophthalmology 2004;111:960-5. [Crossref] [PubMed]
- Queisi MM, Zein M, Lamba N, et al. Update on ocular cicatricial pemphigoid and emerging treatments. Surv Ophthalmol 2016;61:314-7. [Crossref] [PubMed]
- Friedman J, Marcovich AL, Kleinmann G, et al. Low-dose pulsed intravenous cyclophosphamide for severe ocular cicatricial pemphigoid in elderly patients. Cornea 2014;33:1066-70. [Crossref] [PubMed]
- Fehr J, Hofmann V, Kappeler U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N Engl J Med 1982;306:1254-8. [Crossref] [PubMed]
- Okitsu-Negishi S, Furusawa S, Kawa Y, et al. Suppressive effect of intravenous immunoglobulins on the activity of interleukin-1. Immunol Res 1994;13:49-55. [Crossref] [PubMed]
- Menezes MC, Benard G, Sato MN, et al. In vitro inhibitory activity of tumor necrosis factor alpha and interleukin-2 of human immunoglobulin preparations. Int Arch Allergy Immunol 1997;114:323-8. [Crossref] [PubMed]
- Ling ZD, Yeoh E, Webb BT, et al. Intravenous immunoglobulin induces interferon-gamma and interleukin-6 in vivo. J Clin Immunol 1993;13:302-9. [Crossref] [PubMed]
- Ruiz de Souza V, Carreno MP, Kaveri SV, et al. Selective induction of interleukin-1 receptor antagonist and interleukin-8 in human monocytes by normal polyspecific IgG (intravenous immunoglobulin). Eur J Immunol 1995;25:1267-73. [Crossref] [PubMed]
- Frank MM, Basta M, Fries LF. The effects of intravenous immune globulin on complement-dependent immune damage of cells and tissues. Clin Immunol Immunopathol 1992;62:S82-6. [Crossref] [PubMed]
- Wollina U, Koch A, Hansel G. Rituximab therapy of recalcitrant bullous dermatoses. J Dermatol Case Rep 2008;2:4-7. [Crossref] [PubMed]
- Zaki AM, Suhler EB. Biologics in non-infectious uveitis past, present and future. Ann Eye Sci 2021;6:20. [Crossref]
- You C, Lamba N, Lasave AF, et al. Rituximab in the treatment of ocular cicatricial pemphigoid: a retrospective cohort study. Graefes Arch Clin Exp Ophthalmol 2017;255:1221-8. [Crossref] [PubMed]
- Rübsam A, Stefaniak R, Worm M, et al. Rituximab preserves vision in ocular mucous membrane pemphigoid. Expert Opin Biol Ther 2015;15:927-33. [Crossref] [PubMed]
- Wollina U, Pabst F, Kuss H, et al. Monoclonal anti-CD20 Antibody Therapy in Cicatrical Pemphigoid with Oral and Hypopharyngeal Involvement and Related Conditions. J Clin Aesthet Dermatol 2013;6:45-8. [PubMed]
- Heelan K, Walsh S, Shear NH. Treatment of mucous membrane pemphigoid with rituximab. J Am Acad Dermatol 2013;69:310-1. [Crossref] [PubMed]
- Le Roux-Villet C, Prost-Squarcioni C, Alexandre M, et al. Rituximab for patients with refractory mucous membrane pemphigoid. Arch Dermatol 2011;147:843-9. [Crossref] [PubMed]
- Ross AH, Jaycock P, Cook SD, et al. The use of rituximab in refractory mucous membrane pemphigoid with severe ocular involvement. Br J Ophthalmol 2009;93:421-2, 548. [Crossref] [PubMed]
- Lourari S, Herve C, Doffoel-Hantz V, et al. Bullous and mucous membrane pemphigoid show a mixed response to rituximab: experience in seven patients. J Eur Acad Dermatol Venereol 2011;25:1238-40. [Crossref] [PubMed]
- Schumann T, Schmidt E, Booken N, et al. Successful treatment of mucous membrane pemphigoid with the anti-CD-20 antibody rituximab. Acta Derm Venereol 2009;89:101-2. [Crossref] [PubMed]
- Taverna JA, Lerner A, Bhawan J, et al. Successful adjuvant treatment of recalcitrant mucous membrane pemphigoid with anti-CD20 antibody rituximab. J Drugs Dermatol 2007;6:731-2. [PubMed]
- Schmidt E, Seitz CS, Benoit S, et al. Rituximab in autoimmune bullous diseases: mixed responses and adverse effects. Br J Dermatol 2007;156:352-6. [Crossref] [PubMed]
- Bevans SL, Parker J, Ivey JM, et al. Rituximab as an Adjuvant Rescue Treatment for Ocular Cicatricial Pemphigoid. Cornea 2021;40:1440-4. [Crossref] [PubMed]
- Foster SOCPIU. In: Zone JJ. editor. UpToDate. Accessed on June 7, 2022. Available online: https://www.uptodate.com/contents/ocular-cicatricial-pemphigoid?search=foster%20ocular%20cicatricial%20pemphigoid&source=search_result&selectedTitle=1%7E129&usage_type=default&display_rank=1
- Cordero Coma M, Yilmaz T, Foster CS. Tumour necrosis factor-alpha in conjunctivae affected by ocular cicatricial pemphigoid. Acta Ophthalmol Scand 2007;85:753-5. [Crossref] [PubMed]
- Razzaque MS, Foster CS, Ahmed AR. Role of macrophage migration inhibitory factor in conjunctival pathology in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci 2004;45:1174-81. [Crossref] [PubMed]
- John H, Whallett A, Quinlan M. Successful biologic treatment of ocular mucous membrane pemphigoid with anti-TNF-alpha. Eye (Lond) 2007;21:1434-5. [Crossref] [PubMed]
- Canizares MJ, Smith DI, Conners MS, et al. Successful treatment of mucous membrane pemphigoid with etanercept in 3 patients. Arch Dermatol 2006;142:1457-61. [Crossref] [PubMed]
- Prey S, Robert PY, Drouet M, et al. Treatment of ocular cicatricial pemphigoid with the tumour necrosis factor alpha antagonist etanercept. Acta Derm Venereol 2007;87:74-5. [Crossref] [PubMed]
- Sacher C, Rubbert A, König C, et al. Treatment of recalcitrant cicatricial pemphigoid with the tumor necrosis factor alpha antagonist etanercept. J Am Acad Dermatol 2002;46:113-5. [Crossref] [PubMed]
- Heffernan MP, Bentley DD. Successful treatment of mucous membrane pemphigoid with infliximab. Arch Dermatol 2006;142:1268-70. [Crossref] [PubMed]
- Sharon Y, Chu DS. Adrenocorticotropic hormone analogue as novel treatment regimen in ocular cicatricial pemphigoid. Am J Ophthalmol Case Rep 2018;10:264-7. [Crossref] [PubMed]
- Sharon Y, Anesi SD, Martinez CE, et al. Repository Corticotropin Injection as an Alternative Treatment for Refractory Ocular Mucous Membrane Pemphigoid. Cornea 2022;41:45-51. [Crossref] [PubMed]
Cite this article as: Castiblanco C. Ocular cicatricial pemphigoid: diagnosis and systemic management. Ann Eye Sci 2024;9:10.



