
Cell-based therapies for limbal stem cell deficiency: a literature review
Introduction
Background
Limbal stem cell deficiency (LSCD) is characterized by damage to or loss of limbal epithelial stem cells, which maintain the continuous renewal of corneal epithelial cells (1). Limbal epithelial stem cells are located in the basal limbal epithelium, anatomically marked by the Palisades of Vogt located at the cornea-conjunctiva junction. Deficiency in these cells can result in persistent epitheliopathy, conjunctivalization of the cornea, and the loss of corneal transparency and visual function (2). Other LSCD consequences include persistent epithelial defects, corneal opacity, neovascularization, inflammation, ulceration, corneal thinning, and perforation. Several etiologies for LSCD have been introduced, which may be hereditary or acquired in origin. Inherited conditions such as aniridia are rare. Acquired conditions can be categorized as immune-related conditions such as Stevens-Johnson syndrome (SJS), mucus membrane pemphigoid (MMP), and non-immune pathologies like burns or previous multiple ocular surgeries (1,3).
The treatment of LSCD depends on the severity and extent of limbal stem cell loss. Conservative management with lubrication is suited for mild cases, while various surgical interventions can be utilized in severe cases. In unilateral cases, limbal stem cell transplantation is performed using limbal grafts taken from the patient’s healthy second eye [conjunctival limbal autograft (CLAU)]. Autologous limbal stem cell transplants from a patient’s unaffected eye in unilateral LSCD eliminate the need for immunosuppression, but it increases the risk of developing LSCD in the healthy eye. In allogeneic transplantations, limbal grafts are from healthy living-related donors or cadaver corneas. In all allogeneic transplants, systemic immunosuppression is required for a prolonged or indefinite period (1).
Rationale and knowledge gap
More recently, various cell-based therapies have been explored as alternative treatment options. Interestingly, ophthalmology is one of the first medical disciplines to utilize stem cell application in regenerative medicine (4). The overall literature on LSCD and its treatment is growing rapidly and numerous papers are published annually on this topic. So, we aimed to provide and update on the cell-based therapies for LSCD to help clinicians and researchers alike.
Objective
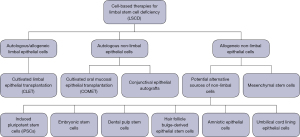
This review discusses the mechanisms and utility of various cell-based therapies such as cultivated limbal epithelial transplantation (CLET) and other non-limbal and stem cell sources for transplantation (Figure 1). The key question to be explored is how cell-based therapies can play a role in LSCD treatment. We present this article in accordance with the Narrative Review reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-22-55/rc).
Methods
The literature search identification process included years starting from 1989, English language, published status, a database of coverage including PubMed, and study designs consisting of original articles, review articles, and case series (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | August 10th, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Limbal stem cell deficiency, cell-based therapy, cultivated limbal epithelial transplantation, cultivated oral mucosal epithelial transplantation, mesenchymal stem cells |
| Timeframe | 1989 onwards |
| Inclusion and exclusion criteria | Inclusion: keywords in English literature from PubMed |
| Exclusion: all other items not included in the inclusion criteria | |
| Selection process | Conducted by Dr. Djalilian, we searched the literature, and a consensus was reached according to author experience and source assessment |
Limbal epithelial cells
CLET was first described in 1997 by Pellegrini et al. They showed that limbal epithelial cells could be cultured ex vivo and give rise to the stratified corneal epithelium. In this procedure, a 1 mm2 biopsy is taken from the healthy eye, cultured ex vivo with various serums and growth factors, and subsequently transplanted on the deficient eye with grafts containing 2×106 cells. It is of note that grafts may have an autologous or allogeneic origin (5).
More recently, CLET has been tested in more extensive studies which have shown reasonable success rates, defined as restoration of a stable, transparent, and avascular cornea. Rama et al. (6) reported a success rate of 68.2% at 1 year in 107 eyes after the first autologous CLET transplantation. Sangwan et al. (7) similarly found a success rate of 71% in a retrospective study of 200 eyes that underwent CLET for LSCD from ocular surface burns. Fasolo et al. (8) also showed a lower success rate of 41% in 65 consecutive CLET transplantations of 59 eyes. However, an additional 39% of cases were classified as partially successful (relapsed neovascularization but not as extensive as admission). In the study by Rama et al., it is found that 11 eyes that were partially successful or failed after primary CLET were re-grafted with 9 of the 11 (81.8%) classified as successful. This resulted in a final success rate of 76.6%. In this study, age, the underlying cause of LSCD, the severity of injury, culturing and postoperative complications, as well as inflammation were found to be associated with failure based on univariate logistic-regression analysis. The severity of injury and culturing and postoperative complications were confirmed on multivariate logistic regression analysis. An interesting finding in this study was the role of p63-bright cell density (representing holoclones which are enriched in stem cells) in the success rate of grafts; cultures containing >3% of p63-bright cells had a success rate of 78% while cultures containing ≤3% of these cells population resulted in the success rate of 11%. This finding shows that >3% p63-bright cell density is necessary but not enough for successful treatment since over 20% of cultures with this property had failed (6).
Basu et al. (9) performed a retrospective study focusing on repeat CLET. These repeat transplants do not increase the risk of rejection or necessitate immunosuppressive treatment given the autologous nature of the transplant and have a success rate of 66% in the 50 eyes included, similar to the rates noted in primary CLET. Most of these eyes undergoing repeat autologous CLET also exhibit improvement in best corrected visual acuity (BCVA) of at least 2 lines.
Autologous CLET transplantation significantly reduces the risk of iatrogenic LSCD in the donor’s eye compared to the larger traditional CLAU transplantations. However, as reported by the Holland group, if the donor’s eyes are carefully screened and selected, the risk of iatrogenic LSCD in the donor’s eye is minimal (10).
Although our knowledge regarding the long-term outcomes of CLET is limited, successful clinical results of up to 10 years (with a median of 2 years) were reported by Rama et al. (6). Regarding the comparison between long-term outcomes of CLET and CLAU, Sharma et al. (11) reported equal effectiveness in restoration of the ocular surface after chemical burns for both procedures. They performed CLET on 10 patients and CLAU in 51 eyes and all of the eyes experienced improvement in ocular surface condition. Although CLET provides more stem cells in the recipient’s eye and avoids iatrogenic LSCD in the donor’s eye, CLAU is cheaper and also can simultaneously address the pre-existing symblepharon (11).
Less data is available regarding clinical outcomes of CLET in children. One study looking at children under 15 years of age who underwent CLET for LSCD due to ocular burns showed a success rate was 37.4% in 107 eyes, with a significantly lower rate of success in children 6 years or younger (30%) compared to those over 6 years of age (70%). Multivariable analyses in this study also found that the survival rates of the grafts were significantly higher in eyes that underwent surgery more than 4 months after the injury. At 24 months, the survival rate was 12.8% in eyes operated at or earlier than 4 months after the initial injury, and 54% in eyes operated on more than 4 months after the injury. This lower success rate compared to the adult trials is likely multifactorial and may partially be attributed to an increased inflammatory response in children. In addition, children also have a much lower likelihood of visual improvement after surgery due to deprivation amblyopia (12).
CLET has also been performed with allografts, especially in cases of bilateral LSCD, although this is less commonly performed than autologous CLET. A recent meta-analysis including 30 studies and 1,306 eyes reported a ratio of 75.2% to 24.7% of eyes had undergone autologous versus allogeneic CLET. No statistically significant difference was found in terms of graft survival and visual improvement. However, autologous CLET, whenever feasible, eliminates the need for long-term immunosuppression (13).
The precise process by which limbal stem cells are cultivated ex vivo and transplanted back onto the human cornea in CLET varies slightly between studies. There are differences in the use of feeder cells, culture media, serum, and scaffolds across these studies. Feeder cells help to create a suitable microenvironment and vary from irradiated or mitomycin-treated murine 3T3 (mitotically inactive) cells to human mesenchymal stem cells and limbal melanocytes. Similarly, animal and human-derived serums and growth products are used for the culture media. A few studies have also investigated serum-free culture protocols (1). The cultured cells are then transported to the diseased cornea using various scaffolds, including human amniotic membrane (HAM), collagen, fibrin, contact lenses, or other hydrogels (1).
In February 2015, CLET transplantation by the name of Holoclar® (Chiesi Farmaceutici SpA, Parma, Italy) was the first stem-cell containing advanced therapy medicinal product (ATMP) to be approved for use in the European Union by the European Medicines Agency and is approved to treat adult patients with moderate or severe LSCD due to burns, including chemical burns. It should be emphasized that at the time being, Holoclar has authorization only for application in cases due to burns with cultured grafts of autologous origin (7,14).
Non-limbal epithelial cells
In cases of total bilateral LSCD, allogeneic transplants are required either from living related donors or cadaveric donors, necessitating long-term chronic immunosuppression and increasing the risk of disease transmission. This has led researchers to seek other alternative non-limbal cells that may be used for autologous grafts. Studies have investigated a variety of non-limbal cells including oral mucosal epithelial cells (15-24), cultivated autologous conjunctival epithelial cells (25-28), induced pluripotent stem cells (iPSCs) (29-38), umbilical cord derived stem cells (39,40), hair follicle derived epithelial stem cells (41,42), dental pulp stem cells (43-46), and nasal mucosal cells (47,48).
Autologous non-limbal epithelial cell transplantation
Cultivated oral mucosal epithelial transplantation (COMET)
Of the non-limbal epithelial cell transplantations studied for the treatment of LSCD, the most well-studied is COMET. This procedure is also called ex vivo cultivated oral mucosal autograft (EVOMAU). COMET was first performed on humans in 2004 by Nakamura et al. (15). Six eyes of 4 patients with bilateral LSCD underwent COMET in this study, showing the survival of the transplanted oral epithelium at 2 days and improvement in visual acuity at follow-up of at least 11 months. Peripheral neovascularization, however, was found in all eyes. It has been reported that COMET is the most common autologous non-limbal epithelial cell transplantation in cases with bilateral LSCD (20). Lack of graft rejection and immunosuppressive regimen may justify this point.
Several subsequent studies evaluating COMET have since been performed (16-19) and COMET has proven to be promising in the treatment of LSCD with a variety of etiologies including ocular burns, aniridia, SJS, and ocular cicatricial pemphigoid (OCP). Cabral et al. performed a meta-analysis of 24 studies and 343 eyes, for which COMET was used to treat various etiologies of LSCD. This study showed a relative success rate defined as a stable ocular surface of 70.8% of 243 eyes (20). Dobrowolski et al. (21) and Venugopal et al. (22) also examined the outcomes of COMET in limbal stem cell deficient eyes exclusively due to aniridia and SJS, respectively. Dobrowolski et al. reported that 76.4% of eyes undergoing COMET for LSCD due to aniridia had a stable corneal epithelial surface after COMET transplantation, with 88% endorsing an improvement in the quality of vision (21). In 45 eyes that underwent COMET for severe SJS, COMET was also shown to be beneficial in 88.8% of eyes with a reduction in LSCD severity scores after the transplant (22). Phenotypic characterization of cells after COMET has also been performed and shows that cells retain markers for oral epithelial cells on impression cytology with immunofluorescence staining, even in successful cases (24,49). In addition, peripheral neovascularization, while not visually significant and reportedly stable at 6 months in the above studies, may continue to progress as oral mucosal cells have greater angiogenic potential compared to limbal epithelial cells (49,50).
While COMET has the main advantage of eliminating the need for chronic immunosuppression, its visual outcomes are inferior to other limbal transplant procedures. This is partly because the oral mucosal epithelium is thicker and more opaque than normal corneal epithelium. A direct comparison study looking at allogeneic CLET and COMET by Wang et al. found a higher incidence of persistent epithelial defect, a worse ocular surface grade, a lower success rate, and a higher risk of graft failure after COMET compared to allogeneic CLET (23). Overall, while there is no consensus on the role of COMET in the management of bilateral LSCD, it may be considered a surface stabilization procedure especially when the patient is not a candidate for immunosuppression.
Conjunctival epithelial autografts
Human conjunctival epithelial cells are another type of non-limbal epithelial cells that have been studied for LCSD (25-28). Ang et al. compared cultivated human corneal epithelial cells and cultivated human conjunctival epithelial cells transplanted into rabbit eyes and found that immunohistochemical analysis of the conjunctival epithelial cells showed similar markers of corneal epithelium. Clinical outcomes were also comparable, with the majority of rabbit eyes demonstrating smooth, transparent corneas without epithelial defects (25). Jeon et al. demonstrated similar findings, showing that the cultivated conjunctival cells can acquire a corneal epithelial phenotype after transplantation (26). Although most studies thus far have been in animal models, Ricardo et al. demonstrate preliminary results of ex vivo-cultivated conjunctival epithelial cells in 12 human eyes with promising results. Re-epithelization with a transparent and regular epithelium without neovascularization was found in 8 of 12 eyes (66.6%) and they also demonstrated visual improvement. Overall, while the results are encouraging and avoid the need for immunosuppression, long-term restoration of the corneal phenotype is not likely with only conjunctival epithelial transplants (without any interventions to restore the limbal niche) and hence many of the problems associated with LSCD are likely to recur over time (28).
Allogeneic non-limbal stem cell-based transplantation
Mesenchymal stem/stromal cells (MSCs)
MSCs are a population of cells that can differentiate into a variety of mesodermal lineage cells including osteoblasts, adipocytes, and chondroblasts. In addition, MSCs secrete various cytokines and growth factors, creating an immunomodulatory and anti-inflammatory environment (51-53). Because of these features, MSCs have become widely studied in a variety of conditions including severe corneal diseases. To date, more than 1,000 clinical trials have been registered using MSCs derived from bone marrow, fat, umbilical cord, and dental pulp.
Multiple animal models have shown promising results with MSCs in the regeneration of the corneal surface in different ocular surface diseases (54-63) as well as in LSCD (64). More recently, a proof-of-concept study was performed comparing allogeneic bone marrow MSC transplantation to allogeneic CLET (65). After 6–12 months, 72.7–77.8% of CLET cases and 76.5–85.7% of MSC cases were successful with no adverse events related to cell products. Central corneal epithelial phenotype was also examined in this study via in vivo confocal microscopy and showed improvement in the epithelial phenotype in both groups with no statistically significant difference. This report is the only clinical application of MSCs in LSCD (65).
Different routes of administration of MSCs have also been studied, including topical administration (56,66), sub-tenon injections, and intravenous injections (55,67,68). While the first human trial utilized HAMs as a carrier for the delivery of stem cells, Galindo et al. also showed promising results in utilizing subconjunctival injections to deliver MSCs (69). This study used an animal model, which may be a good alternative for clinical use in the future.
MSCs may have several benefits compared to limbal epithelial cells, including harvesting from multiple tissues, independency from cadaveric donors, as well as a faster and cheaper process. Moreover, 100% of the MSCs in a transplant are stem cells, whereas the population of stem cells in CLET may be extremely smaller (70). It seems that usage of MSCs in the treatment of LSCD is safe without subsequent adverse reactions, toxicity, and tumorigenicity (54,67,71-79). Currently, we are conducting a clinical trial to evaluate the safety and maximally tolerated dose of locally delivered allogeneic MSCs. In this study, different doses of bone marrow-derived MSCs were used in the route of subconjunctival injection to evaluate safety as well as anatomical and functional results in adult cases of neurotrophic keratitis (80). The results of the first three patients were reported in the annual ARVO 2022 meeting.
Alternative potential sources of non-limbal cells
In recent years, new promising options have been investigated and studied (81).
iPSCs
iPSCs have been at the forefront of regenerative medicine and investigated as another possible treatment modality in LSCD (29,30). iPSCs can be induced from differentiated cells and reprogrammed into a variety of cells including corneal epithelial cells. Over the years, researchers have successfully transitioned from using mouse models (31,82) to human models (32,33). Mikhailova et al. have more recently studied iPSC-derived limbal epithelial stem cells and showed that they are able to retain their capacity for proliferation on bioengineered collagen matrices (34). Additional protocols and studies are also promising and suggest that human pluripotent stem cell-derived limbal stem cells may become a valuable cell source for treatments in the near future (35-38). Currently, more work needs to be done before this method can be implemented in large-scale clinical trials. Recently, a team of scientists from Osaka University reported the results of a world-1st trial on iPSC-based corneal transplantation (83). They performed this trial successfully on 4 patients without any rejection or tumorigenicity.
It should be mentioned that this technique carries a considerable expense, is time-consuming, and concerns about immune-related problems remain. HLA-typed IPSC banks can be a solution to these issues (84,85). Moreover, rigorous genetic analysis should be performed before transplantation to ascertain the lack of mutagenicity (86).
Embryonic stem cells
Human embryonic stem cells (hESCs) are pluripotent stem cells with the capability of differentiation into corneal and limbal epithelial cells (87). Hence, application of these cells may be beneficial in LSCD. Although challenging, several in vitro models have been successfully used to differentiate hESCs into corneal epithelial-like cells (88-92). Nevertheless, ethical challenges and potential immunogenicity and tumorigenicity may act as possible limiting factors (93).
Dental pulp stem cells
A similarity between marker expression of immature human dental pulp stem cells, mesenchymal stem cells, embryonic stem cells, and limbal epithelial stem cells has been shown. The success of transplantation of these stem cells in restoration of ocular surface structure and diminishing pathological features such as conjunctivalization and corneal neovascularization has been reported in animal LSCD models (43,44). Further studies are needed to investigate the safety and efficacy of this before clinical application.
Hair follicle bulge-derived epithelial stem cells
By providing a limbal-like microenvironment with a special culture medium, stem cells harvested from hair follicles may differentiate into corneal epithelial-like cells (94). Although this source of stem cells has been used successfully in a mouse LSCD model (41), our knowledge regarding this technique is limited.
Amniotic membrane epithelial cells
It seems that expressed markers of amniotic membrane epithelial cells have a significant overlap with mesenchymal and embryonic stem cells. The other advantage of these cells is in showing immunomodulatory characteristics (95,96). In rabbit models, these cells have been successfully applied to treat LSCD (97-100).
Umbilical cord lining epithelial cells
Human umbilical cord lining epithelial cells can be considered another potential source for the management of LSCD. Animal models using this type of stem cells are available in the literature (39). Lack of mutagenicity and low immunogenicity are among the advantages of these cells (101).
The strengths of this review are describing the success rates, and complications of different cell-based therapies for LSCD and also discussing each therapy’s relative strengths and weaknesses, their history in animal and human studies, and their effectiveness compared to traditional transplants.
Conclusions
Cell-based therapies are a promising treatment modality for both unilateral and bilateral limbal stem cell deficiency. While CLET is currently the only approved cell-based therapy and is only approved in the European Union, more novel methods have also been shown to be effective in human or animal studies thus far. Non-limbal epithelial cells such as cultivated oral mucosal cells (COMET) are also an alternative treatment to allogeneic transplants especially as a surface stabilizing procedure. Induced pluripotent stem cells are currently being studied in early phase trials and have the potential to revolutionize the way LSCD is treated. Lastly, cell-based therapies for restoring the limbal niche such as mesenchymal stem cells have also shown promising results in the first human proof-of-concept study. Several potential sources of non-limbal cells are under investigation.
Acknowledgments
We would like to express our special thanks to Collin Chow and Khandaker N. Anwar for their effort in assisting with the preparation of this report.
Funding: This work was supported by the National Eye Institute/National Institutes of Health and the Core Grant for Vision Research [R01 EY024349 (ARD), UH3 EY031809 (ARD), EY01792]; Department of Defense Vision Research Program – Congressionally Directed Medical Research Program [VR170180]; Research to Prevent Blindness Unrestricted Grant to the department and Physician-Scientist Award.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Joann Kang and Roy S. Chuck) for the series “Ocular Surface Reconstruction/Transplantation” published in Annals of Eye Science. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aes.amegroups.com/article/view/10.21037/aes-22-55/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-22-55/coif). The series “Ocular Surface Reconstruction/Transplantation” was commissioned by the editorial office without any funding or sponsorship. ARD declares this work was supported by the National Eye Institute/National Institutes of Health and the Core Grant for Vision Research [R01 EY024349 (ARD), UH3 EY031809 (ARD), EY01792]; Department of Defense Vision Research Program – Congressionally Directed Medical Research Program [VR170180]; Research to Prevent Blindness Unrestricted Grant to the department and Physician-Scientist Award. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elhusseiny AM, Soleimani M, Eleiwa TK, et al. Current and Emerging Therapies for Limbal Stem Cell Deficiency. Stem Cells Transl Med 2022;11:259-68. [Crossref] [PubMed]
- Le Q, Chauhan T, Cordova D, et al. Biomarkers of in vivo limbal stem cell function. Ocul Surf 2022;23:123-30. [Crossref] [PubMed]
- Schlötzer-Schrehardt U, Latta L, Gießl A, et al. Dysfunction of the limbal epithelial stem cell niche in aniridia-associated keratopathy. Ocul Surf 2021;21:160-73. [Crossref] [PubMed]
- Figueiredo FC, Glanville JM, Arber M, et al. A systematic review of cellular therapies for the treatment of limbal stem cell deficiency affecting one or both eyes. Ocul Surf 2021;20:48-61. [Crossref] [PubMed]
- Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997;349:990-3. [Crossref] [PubMed]
- Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010;363:147-55. [Crossref] [PubMed]
- Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol 2011;95:1525-9. [Crossref] [PubMed]
- Fasolo A, Pedrotti E, Passilongo M, et al. Safety outcomes and long-term effectiveness of ex vivo autologous cultured limbal epithelial transplantation for limbal stem cell deficiency. Br J Ophthalmol 2017;101:640-9. [Crossref] [PubMed]
- Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol 2012;153:643-50, 650.e1-2.
- Cheung AY, Sarnicola E, Holland EJ. Long-Term Ocular Surface Stability in Conjunctival Limbal Autograft Donor Eyes. Cornea 2017;36:1031-5. [Crossref] [PubMed]
- Sharma R, Sharma A, Nirankari VS. Cultivated Limbal Epithelial Transplant Versus Conjunctival Limbal Auto Transplant in Uniocular Limbal Stem Cell Deficiency: Long-Term Results. Medical Research Archives 2022;10: [Crossref]
- Sejpal K, Ali MH, Maddileti S, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol 2013;131:731-6. [Crossref] [PubMed]
- Mishan MA, Yaseri M, Baradaran-Rafii A, et al. Systematic review and meta-analysis investigating autograft versus allograft cultivated limbal epithelial transplantation in limbal stem cell deficiency. Int Ophthalmol 2019;39:2685-96. [Crossref] [PubMed]
- Pellegrini G, Ardigò D, Milazzo G, et al. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl Med 2018;7:146-54. [Crossref] [PubMed]
- Nakamura T, Inatomi T, Sotozono C, et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol 2004;88:1280-4. [Crossref] [PubMed]
- Satake Y, Higa K, Tsubota K, et al. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology 2011;118:1524-30. [Crossref] [PubMed]
- Satake Y, Yamaguchi T, Hirayama M, et al. Ocular surface reconstruction by cultivated epithelial sheet transplantation. Cornea 2014;33:S42-6. [Crossref] [PubMed]
- Sotozono C, Inatomi T, Nakamura T, et al. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology 2013;120:193-200. [Crossref] [PubMed]
- Komai S, Inatomi T, Nakamura T, et al. Long-term outcome of cultivated oral mucosal epithelial transplantation for fornix reconstruction in chronic cicatrising diseases. Br J Ophthalmol 2022;106:1355-62. [Crossref] [PubMed]
- Cabral JV, Jackson CJ, Utheim TP, et al. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: a review. Stem Cell Res Ther 2020;11:301. [Crossref] [PubMed]
- Dobrowolski D, Orzechowska-Wylegala B, Wowra B, et al. Cultivated Oral Mucosa Epithelium in Ocular Surface Reconstruction in Aniridia Patients. Biomed Res Int 2015;2015:281870. [Crossref] [PubMed]
- Venugopal R, Nagpal R, Mohanty S, et al. Outcomes of Cultivated Oral Mucosal Epithelial Transplantation in Eyes With Chronic Stevens-Johnson Syndrome Sequelae. Am J Ophthalmol 2021;222:82-91. [Crossref] [PubMed]
- Wang J, Qi X, Dong Y, et al. Comparison of the efficacy of different cell sources for transplantation in total limbal stem cell deficiency. Graefes Arch Clin Exp Ophthalmol 2019;257:1253-63. [Crossref] [PubMed]
- Prabhasawat P, Chirapapaisan C, Jiravarnsirikul A, et al. Phenotypic Characterization of Corneal Epithelium in Long-Term Follow-Up of Patients Post-Autologous Cultivated Oral Mucosal Epithelial Transplantation. Cornea 2021;40:842-50. [Crossref] [PubMed]
- Ang LP, Tanioka H, Kawasaki S, et al. Cultivated human conjunctival epithelial transplantation for total limbal stem cell deficiency. Invest Ophthalmol Vis Sci 2010;51:758-64. [Crossref] [PubMed]
- Jeon S, Choi SH, Wolosin JM, et al. Regeneration of the corneal epithelium with conjunctival epithelial equivalents generated in serum- and feeder-cell-free media. Mol Vis 2013;19:2542-50. [PubMed]
- Silber PC, Ricardo JR, Cristovam PC, et al. Conjunctival epithelial cells cultivated ex vivo from patients with total limbal stem cell deficiency. Eur J Ophthalmol 2014; Epub ahead of print. [Crossref] [PubMed]
- Ricardo JR, Cristovam PC, Filho PA, et al. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea 2013;32:221-8. [Crossref] [PubMed]
- Casaroli-Marano RP, Nieto-Nicolau N, Martínez-Conesa EM, et al. Potential Role of Induced Pluripotent Stem Cells (IPSCs) for Cell-Based Therapy of the Ocular Surface. J Clin Med 2015;4:318-42. [Crossref] [PubMed]
- Zhu J, Slevin M, Guo BQ, et al. Induced pluripotent stem cells as a potential therapeutic source for corneal epithelial stem cells. Int J Ophthalmol 2018;11:2004-10. [PubMed]
- Notara M, Hernandez D, Mason C, et al. Characterization of the phenotype and functionality of corneal epithelial cells derived from mouse embryonic stem cells. Regen Med 2012;7:167-78. [Crossref] [PubMed]
- Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One 2012;7:e45435. [Crossref] [PubMed]
- Sareen D, Saghizadeh M, Ornelas L, et al. Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Transl Med 2014;3:1002-12. [Crossref] [PubMed]
- Mikhailova A, Ilmarinen T, Ratnayake A, et al. Human pluripotent stem cell-derived limbal epithelial stem cells on bioengineered matrices for corneal reconstruction. Exp Eye Res 2016;146:26-34. [Crossref] [PubMed]
- Hayashi R, Ishikawa Y, Sasamoto Y, et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016;531:376-80. [Crossref] [PubMed]
- Hayashi R, Ishikawa Y, Katori R, et al. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nat Protoc 2017;12:683-96. [Crossref] [PubMed]
- Hongisto H, Vattulainen M, Ilmarinen T, et al. Efficient and Scalable Directed Differentiation of Clinically Compatible Corneal Limbal Epithelial Stem Cells from Human Pluripotent Stem Cells. J Vis Exp 2018;58279. [PubMed]
- Vattulainen M, Ilmarinen T, Viheriälä T, et al. Corneal epithelial differentiation of human pluripotent stem cells generates ABCB5(+) and ∆Np63α(+) cells with limbal cell characteristics and high wound healing capacity. Stem Cell Res Ther 2021;12:609. [Crossref] [PubMed]
- Reza HM, Ng BY, Gimeno FL, et al. Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev Rep 2011;7:935-47. [Crossref] [PubMed]
- Ang LP, Jain P, Phan TT, et al. Human Umbilical Cord Lining Cells as Novel Feeder Layer for Ex Vivo Cultivation of Limbal Epithelial Cells. Invest Ophthalmol Vis Sci 2015;56:4697-704. [Crossref] [PubMed]
- Meyer-Blazejewska EA, Call MK, Yamanaka O, et al. From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 2011;29:57-66. [Crossref] [PubMed]
- Call M, Meyer EA, Kao WW, et al. Murine Hair Follicle Derived Stem Cell Transplantation onto the Cornea Using a Fibrin Carrier. Bio Protoc 2018;8:e2849. [Crossref] [PubMed]
- Monteiro BG, Serafim RC, Melo GB, et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif 2009;42:587-94. [Crossref] [PubMed]
- Gomes JA, Geraldes Monteiro B, Melo GB, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci 2010;51:1408-14. [Crossref] [PubMed]
- Patil S, D'Souza C, Patil P, et al. Culture and characterization of human dental pulp derived stem cells as limbal stem cells for corneal damage repair. Mol Med Rep 2019;20:4688-94. [Crossref] [PubMed]
- Monteiro BG, Loureiro RR, Cristovam PC, et al. Amniotic membrane as a biological scaffold for dental pulp stem cell transplantation in ocular surface reconstruction. Arq Bras Oftalmol 2019;82:32-7. [Crossref] [PubMed]
- Kim JH, Chun YS, Lee SH, et al. Ocular surface reconstruction with autologous nasal mucosa in cicatricial ocular surface disease. Am J Ophthalmol 2010;149:45-53. [Crossref] [PubMed]
- Kobayashi M, Nakamura T, Yasuda M, et al. Ocular surface reconstruction with a tissue-engineered nasal mucosal epithelial cell sheet for the treatment of severe ocular surface diseases. Stem Cells Transl Med 2015;4:99-109. [Crossref] [PubMed]
- Chen HC, Yeh LK, Tsai YJ, et al. Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Invest Ophthalmol Vis Sci 2012;53:5615-23. [Crossref] [PubMed]
- Kanayama S, Nishida K, Yamato M, et al. Analysis of angiogenesis induced by cultured corneal and oral mucosal epithelial cell sheets in vitro. Exp Eye Res 2007;85:772-81. [Crossref] [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J 2018;18:e264-77. [Crossref] [PubMed]
- Liang L, Luo X, Zhang J, et al. Safety and feasibility of subconjunctival injection of mesenchymal stem cells for acute severe ocular burns: A single-arm study. Ocul Surf 2021;22:103-9. [Crossref] [PubMed]
- Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells 2006;24:315-21. [Crossref] [PubMed]
- Lan Y, Kodati S, Lee HS, et al. Kinetics and function of mesenchymal stem cells in corneal injury. Invest Ophthalmol Vis Sci 2012;53:3638-44. [Crossref] [PubMed]
- Cejkova J, Trosan P, Cejka C, et al. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res 2013;116:312-23. [Crossref] [PubMed]
- Espandar L, Caldwell D, Watson R, et al. Application of adipose-derived stem cells on scleral contact lens carrier in an animal model of severe acute alkaline burn. Eye Contact Lens 2014;40:243-7. [Crossref] [PubMed]
- Bu P, Vin AP, Sethupathi P, et al. Effects of activated omental cells on rat limbal corneal alkali injury. Exp Eye Res 2014;121:143-6. [Crossref] [PubMed]
- Acar U, Pinarli FA, Acar DE, et al. Effect of allogeneic limbal mesenchymal stem cell therapy in corneal healing: role of administration route. Ophthalmic Res 2015;53:82-9. [Crossref] [PubMed]
- Ke Y, Wu Y, Cui X, et al. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS One 2015;10:e0119725. [Crossref] [PubMed]
- Holan V, Trosan P, Cejka C, et al. A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl Med 2015;4:1052-63. [Crossref] [PubMed]
- Lee MJ, Ko AY, Ko JH, et al. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther 2015;23:139-46. [Crossref] [PubMed]
- Cejka C, Cejkova J, Trosan P, et al. Transfer of mesenchymal stem cells and cyclosporine A on alkali-injured rabbit cornea using nanofiber scaffolds strongly reduces corneal neovascularization and scar formation. Histol Histopathol 2016;31:969-80. [PubMed]
- Li G, Zhang Y, Cai S, et al. Human limbal niche cells are a powerful regenerative source for the prevention of limbal stem cell deficiency in a rabbit model. Sci Rep 2018;8:6566. [Crossref] [PubMed]
- Calonge M, Pérez I, Galindo S, et al. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl Res 2019;206:18-40. [Crossref] [PubMed]
- Zeppieri M, Salvetat ML, Beltrami AP, et al. Human adipose-derived stem cells for the treatment of chemically burned rat cornea: preliminary results. Curr Eye Res 2013;38:451-63. [Crossref] [PubMed]
- Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing. Eye (Lond) 2006;20:482-90. [Crossref] [PubMed]
- Mittal SK, Omoto M, Amouzegar A, et al. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports 2016;7:583-90. [Crossref] [PubMed]
- Galindo S, de la Mata A, López-Paniagua M, et al. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: state of the art. Stem Cell Res Ther 2021;12:60. [Crossref] [PubMed]
- Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 1989;57:201-9. [Crossref] [PubMed]
- Ahmed SK, Soliman AA, Omar SM, et al. Bone Marrow Mesenchymal Stem Cell Transplantation in a Rabbit Corneal Alkali Burn Model (A Histological and Immune Histo-chemical Study). Int J Stem Cells 2015;8:69-78. [Crossref] [PubMed]
- Reinshagen H, Auw-Haedrich C, Sorg RV, et al. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol 2011;89:741-8. [Crossref] [PubMed]
- Galindo S, Herreras JM, López-Paniagua M, et al. Therapeutic Effect of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Experimental Corneal Failure Due to Limbal Stem Cell Niche Damage. Stem Cells 2017;35:2160-74. [Crossref] [PubMed]
- Zajicova A, Pokorna K, Lencova A, et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transplant 2010;19:1281-90. [Crossref] [PubMed]
- Rengasamy M, Gupta PK, Kolkundkar U, et al. Preclinical safety & toxicity evaluation of pooled, allogeneic human bone marrow-derived mesenchymal stromal cells. Indian J Med Res 2016;144:852-64. [Crossref] [PubMed]
- Guess AJ, Daneault B, Wang R, et al. Safety Profile of Good Manufacturing Practice Manufactured Interferon γ-Primed Mesenchymal Stem/Stromal Cells for Clinical Trials. Stem Cells Transl Med 2017;6:1868-79. [Crossref] [PubMed]
- Gramlich OW, Burand AJ, Brown AJ, et al. Cryopreserved Mesenchymal Stromal Cells Maintain Potency in a Retinal Ischemia/Reperfusion Injury Model: Toward an off-the-shelf Therapy. Sci Rep 2016;6:26463. [Crossref] [PubMed]
- Tappenbeck N, Schröder HM, Niebergall-Roth E, et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019;21:546-60. [Crossref] [PubMed]
- Labrador Velandia S, Di Lauro S, Alonso-Alonso ML, et al. Biocompatibility of intravitreal injection of human mesenchymal stem cells in immunocompetent rabbits. Graefes Arch Clin Exp Ophthalmol 2018;256:125-34. [Crossref] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04626583
- Delic NC, Cai JR, Watson SL, et al. Evaluating the clinical translational relevance of animal models for limbal stem cell deficiency: A systematic review. Ocul Surf 2022;23:169-83. [Crossref] [PubMed]
- Yu D, Chen M, Sun X, et al. Differentiation of mouse induced pluripotent stem cells into corneal epithelial-like cells. Cell Biol Int 2013;37:87-94. [Crossref] [PubMed]
- Available online: https://english.kyodonews.net/news/2022/04/c8af6b7913b2-japan-team-proves-ips-based-cornea-transplant-safe-in-world-1st-trial.html
- Taylor CJ, Peacock S, Chaudhry AN, et al. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 2012;11:147-52. [Crossref] [PubMed]
- de Rham C, Villard J. Potential and limitation of HLA-based banking of human pluripotent stem cells for cell therapy. J Immunol Res 2014;2014:518135. [Crossref] [PubMed]
- Sullivan S, Stacey GN, Akazawa C, et al. Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen Med 2018;13:859-66. [Crossref] [PubMed]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7. [Crossref] [PubMed]
- Hanson C, Hardarson T, Ellerström C, et al. Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro. Acta Ophthalmol 2013;91:127-30. [Crossref] [PubMed]
- Zhu J, Zhang K, Sun Y, et al. Reconstruction of functional ocular surface by acellular porcine cornea matrix scaffold and limbal stem cells derived from human embryonic stem cells. Tissue Eng Part A 2013;19:2412-25. [Crossref] [PubMed]
- da Mata Martins TM, da Silva Cunha P, Rodrigues MA, et al. Epithelial basement membrane of human decellularized cornea as a suitable substrate for differentiation of embryonic stem cells into corneal epithelial-like cells. Mater Sci Eng C Mater Biol Appl 2020;116:111215. [Crossref] [PubMed]
- Ahmad S, Stewart R, Yung S, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells 2007;25:1145-55. [Crossref] [PubMed]
- Zhang C, Du L, Pang K, et al. Differentiation of human embryonic stem cells into corneal epithelial progenitor cells under defined conditions. PLoS One 2017;12:e0183303. [Crossref] [PubMed]
- Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest 2010;120:51-9. [Crossref] [PubMed]
- Blazejewska EA, Schlötzer-Schrehardt U, Zenkel M, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 2009;27:642-52. [Crossref] [PubMed]
- Miki T, Lehmann T, Cai H, et al. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005;23:1549-59. [Crossref] [PubMed]
- Miki T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am J Reprod Immunol 2018;80:e13003. [Crossref] [PubMed]
- He YG, Alizadeh H, Kinoshita K, et al. Experimental transplantation of cultured human limbal and amniotic epithelial cells onto the corneal surface. Cornea 1999;18:570-9. [Crossref] [PubMed]
- Fatimah SS, Ng SL, Chua KH, et al. Value of human amniotic epithelial cells in tissue engineering for cornea. Hum Cell 2010;23:141-51. [Crossref] [PubMed]
- Yao M, Chen J, Yang XX, et al. Differentiation of human amniotic epithelial cells into corneal epithelial-like cells in vitro. Int J Ophthalmol 2013;6:564-72. [PubMed]
- Zhou Q, Liu XY, Ruan YX, et al. Construction of corneal epithelium with human amniotic epithelial cells and repair of limbal deficiency in rabbit models. Hum Cell 2015;28:22-36. [Crossref] [PubMed]
- Calonge M, Nieto-Miguel T, de la Mata A, et al. Goals and Challenges of Stem Cell-Based Therapy for Corneal Blindness Due to Limbal Deficiency. Pharmaceutics 2021;13:1483. [Crossref] [PubMed]
Cite this article as: Chen K, Soleimani M, Koganti R, Cheraqpour K, Habeel S, Djalilian AR. Cell-based therapies for limbal stem cell deficiency: a literature review. Ann Eye Sci 2023;8:6.


