
Psychophysics in the ophthalmological practice—II. Contrast sensitivity
Introduction
Contrast is the difference between the luminance of two objects. For example, a dark gray ball on a white tablecloth has high contrast; if the object were light gray, the contrast would be lower.
The definition of contrast can be formalized by the equation:
where Lmax and Lminare the maximum and minimum luminance of an image.
If the background luminance (in our example the tablecloth) is constant, Weber’s law can be assumed.
where L is the luminance of the object and Lback is the luminance of the background (or pedestal, in our case the tablecloth). Snellen optotypes for visual acuity testing have generally Weber contrast ≥90% (1).
The contrast threshold, therefore, is the just noticeable difference (jnd) in luminance. Contrast sensitivity (CS), the reciprocal of contrast threshold (CS =1/contrast threshold), increases as a function of the ability of the observer to perceive this difference.
As it often happens for psychophysical measurement units, CS can be expressed as a logarithmic scale to ensure differences in signal intensity have the same value across the whole spectrum of luminance.
The contrast sensitivity function (CSF)
CS can be measured by using grating stimuli. It is well known that the size of the stimulus affects CS (2): in a grating made of dark and light bars, as the thickness of the bars decreases (the spatial frequency of the grating increases), the amount of contrast required to see the grating increases. It is worth recalling that for a serial stimulus, the spatial frequency corresponds to the number of repetitions per visual space unit (cycles /degree). E.g., a 1 degree-wide grating made of 10 black bars and 10 white bars has a spatial frequency of 10 cycles/deg, where 1 cycle is made up of a white bar plus a black bar.
Luminance change between the stripes is described by a square- or sine-wave function. In the first case, the luminance of the bars is constant across their extension, in the second, luminance is at its maximum along the central axis of the white stripes and decreases progressively towards the extremes, reaching the minimum along the central axis of the dark stripes (Figure 1).
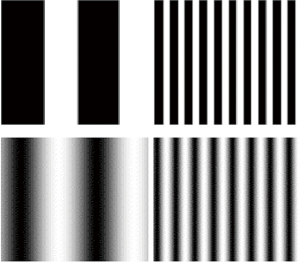
Contrast threshold is therefore the minimum amount of contrast required to detect bars of a given spatial frequency. Since CS is the reciprocal of contrast threshold, the CSF is a threshold function that describes how contrast threshold changes with spatial frequency. It is the result of multidimensional analysis since it describes how a dependent variable (sensitivity) varies as a function of the independent variable (spatial frequency). Conversely, the frequency-of-seeing curve, a sigmoid function that describes the subject’s response to stimulus intensities, is unidimensional and represents the percent of correct responses as a function of the variable under examination (the strength of the signal).
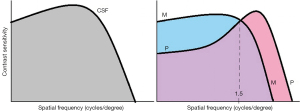
The CSF (Figure 2) can be described by a log parabola with a peak at the medium frequencies (3–6 c/deg) and decay at the high and low spatial frequencies (3,4).

In the absence of alterations of the eye and/or the visual pathway, the fall-off in sensitivity at high spatial frequencies depends on the density of the foveal photoreceptors, thereby on the maximum resolution of the visual system; indeed, the foveal cone system is characterized by higher resolution as well as higher activation threshold than the peripheral retina. The decay at low spatial frequencies, arguably, depends on lateral inhibition between contiguous ganglion cells (the interaction between the opposing signals of center and surround of neighboring detectors leads to spatial attenuation of slow luminance gradients). Therefore, with the CSF, it is possible to estimate the visual acuity, which corresponds to the cut-off frequency represented by the point at which the function intercepts the abscissa, namely the finest gratings that can be detected at 100% contrast.
P-mediated and M-mediated CSF
The anatomofunctional organization of the visual system relies on two cellular pathways: the magnocellular and the parvocellular system (M- and P-system, respectively). The M-system is responsible for the analysis of global and rapidly moving configurations: in effect, it is particularly sensitive to stimuli made of components with low spatial and high temporal frequencies, even more, if at low luminance levels (5,6). On the other hand, it is less activated by high spatial frequencies and low temporal frequencies (7). As an example, consider large clear and dark bars that quickly translate or invert polarity, generating a flicker. Damage to the M-lateral geniculate cells determines reduced CS for stimuli with low spatial frequency (1 cycle/degree) and high temporal frequencies (10 Hz or higher) (7). The P-system is sensitive to static details, therefore it is responsive to high spatial frequencies and low temporal frequencies, particularly at high luminance levels (e.g., a stationary grating made up of narrow dark and light stripes).
The CS curve shown in Figure 2 and Figure 3 is the result of the complementary activity of the M- and P-system. The cut-off or transition point from M/P activity is located between 0.2 and 3.5 cycles/degree (8) or at about 1.5 cycles/degree (9).
Even though the detection of a sine-wave grating at low spatial frequency is carried out by the M-cells, the P-system is globally more responsive, so its activation extends to a certain degree to the M-domain. Maunsell and colleagues, for example, found a moderate parvocellular activation in the middle temporal visual area, responsible for processing M-mediated motion perception (10). For this reason, an impairment of the P-system decreases CS not only at the high spatial frequencies, as expected, but to some extent even at the low frequencies [1 cycle/degree (11,12); 2 cycles/degree (13)]. In fact, Merigan and Eskin observed a deficit of CS for stationary gratings up to 0.5 cycles/degree after selective destruction of the geniculate parvocellular layers in monkeys. However, the deficit was less evident when the temporal frequency was increased, as the result of a greater M-activation (14). The response is saturated at spatial frequencies of about 1 cycle/degree and temporal frequency of 10 Hz: spatial frequencies of less than 1 cycle/degree with a temporal frequency of at least 10 Hz, at low luminance levels, can be considered, with due caution, the best experimental parameters to estimate the magnocellular activity.
Types of CS loss in patients
By examining the CSF, four patterns of alteration can be identified (15):
- A defect limited to the high spatial frequencies that reflects a reduction of visual acuity as commonly measured with high-contrast symbols; this defect is typical of uncorrected ametropia or amblyopia (16,17);
- A defect that encompasses all the frequencies. In this case, low CS at the higher spatial frequencies is responsible for reduced visual acuity and is associated with a deficit at medium and low spatial frequencies. This is typical of lenticular and neural aging (18,19);
- A defect limited to the mid-range frequencies as in the case of multiple sclerosis (20);
- A defect to the medium-low spatial frequencies in case of glaucoma, optic neuritis, papilledema, multiple sclerosis, or diabetes (21,22), or at the low spatial frequencies in dyslexia, as postulated by the so-called magnocellular theory. A class of dyslexics struggle to read because of a deficit in the dorsal magnocellular visual pathway [see (23) for a review on this topic]. In line with this, some studies found reduced CS at low spatial frequencies in these patients (24,25), especially at high temporal frequencies and meso-scotopic conditions, i.e., those mediated by the magnocellular system (26). Nevertheless, the finding is arguable, as the parvocellular system contributes significantly to contrast threshold at all the frequencies (27).
To plot accurately the threshold function, the entire bandwidth should be evaluated: yet, it is time-consuming, especially within the clinical setting. To overcome this problem and quickly categorize the CS loss, Cobo-Lewis (28) proposed an adaptive Bayesian procedure based on the so-called Minimum Estimated Expected Entropy (MEEE).
To establish if the visual function is normal, estimating as few as two functional parameters is stated to be sufficient, namely CS at high spatial frequencies, that reflects visual acuity, and at mid-range spatial frequencies (29). The combination of these two measurements, indeed, provides a good estimate of the CSF with a reduced number of trials.
Clinical assessment of CS
The first rudimentary test to investigate CS was introduced in the 18th century by Boguer. It consisted of two candles, one placed near a screen and the other farther away. An opaque bar positioned between the two candles at a variable distance cast a shadow on the screen. The amount of contrast was given by the differential luminance between the background (the screen) and the shadow (the target). The bar was moved away until the patient was no longer able to detect the shadow. In the 19th century, Bjerrum introduced the first low contrast optotype (alphanumeric stimuli with a contrast of 9%, 20%, 30%, and 40%). Later on, in the 1950s, Fortuin adopted optotypes with various levels of luminance. Finally, in the 1960s, sine-wave gratings started to be used (4) [for a historical review of the tests see (30)].
Since then, more rigorous and sophisticated exams for the measurement of CS have been developed, like optotypic tables or psychophysical algorithms implemented by computerized techniques.
The Pelli-Robson CS-chart
The Pelli-Robson chart (29) depicts lines of letters invariant in size but changing in contrast, expressed as Weber’s fraction (Figure 4). According to the authors, this solution is preferable, since the recognition of letters is a more familiar task than the detection of gratings. This table is calibrated for a distance of 3 meters and is made up of lines of letters 0.5 degrees wide (Sloan font) arranged one under the other. Presenting the table at 1 meter is especially suitable to test low-vision patients: at this viewing distance the letters subtend 2.8 degrees of visual angle.
Each line consists of two triplets of letters: the contrast of the right triplet is lower than the left one by a factor of 1/√2 (i.e., 0.15 log units). Contrast is reduced by the same amount not only between the two triplets of the line but also across the lines. The observer is asked to read each line from the top to the bottom of the chart. Contrast threshold is computed as the amount of contrast of the triplet preceding the one in which two letters have not been recognized. As for visual acuity, the exam does not include the presentation of all the stimuli on the table but ends when the observer is no longer able to recognize two letters of the triplet.
Pelli estimated that letters 0.5 deg in size are suitable for measuring CS at spatial frequencies between 3 and 5 cycles/degree, i.e., at the medium frequencies around the middle of the CSF, which the human visual system is optimally sensitive to. He pinpointed that this bandwidth provides as much information on CS as would be clinically useful. For this reason, in association with the measurement of visual acuity, the Pelli-Robson chart is enough to characterize the global visual function of the subject. The target probability for threshold estimation depends on the response model. Assuming it is 26-AFC (i.e., as many alternatives as the letters of the alphabet, even if, unknown to the observer, only 10 characters for the Sloan font are presented), implicit version [for a definition of standard- and implicit-AFC version see (31)], the guess rate is 3.8%. Therefore, a target probability higher than (100% + 3.8%)/2 =51.9% is required. The recognition of 2 out of three characters of the triplet means a 66% proportion of correct responses. A procedure similar to the Pelli-Robson is the Mars Letter Contrast Sensitivity Test. The Mars Letter Contrast Sensitivity Test (32) is made of a set of three alphanumeric charts (one for the right eye, one for the left eye, and one for binocular vision) calibrated for near vision distance. Like in the Pelli-Robson chart, the size of the symbols is constant. In each table, the contrast level is progressively reduced letter-by-letter by 0.04 log unit steps (48 contrast levels) and the score is computed from the letter (or number) with the smallest amount of contrast that can be perceived in each table.
The Low-Contrast Sloan Letter Charts (LCSLCs)
The Pelli-Robson charts measure CS of letters with a predetermined size. In turn, the LCSLCs (33) estimate visual acuity at a certain amount of contrast. Seven tables (illuminance: 861-1076 lux) with lines of Sloan letters in ETDRS-like format are administered at a distance of 2 meters. The seven tables differ in contrast, from 100% to 0.6% (Figure 5). The subject is asked to read the letters in each table starting from the upper line, then an acuity score corresponding to the number of letters identified correctly is computed for each contrast level (the scoring system is similar to the letter-by-letter ETDRS method for the assessment of visual acuity).

As the contrast of the table is lower, the recognition task becomes more and more demanding. Therefore, the letter score for each chart is inversely correlated to the visual threshold at that contrast value: the higher the score, the higher the sensitivity at that contrast level. The psychophysical procedure resembles that of Pelli-Robson (26-AFC response model, implicit variant, method of constant stimuli) but differs since, as explained, the LCSLCs measure visual acuity as a function of a given amount of contrast: so, the LCSLCs do not directly estimate CS, but the effect of contrast on visual acuity.
The Arden gratings (grating CS test)
The Arden test (34) makes use of 6 panels with luminance 130–150 cd·m-2, each reproducing a sine-wave grating with different spatial frequencies (0.2, 0.4, 0.8, 1.6, 3.2, 6.4 c/deg). The contrast of the gratings increases across the panel from top to bottom by a value equal to 0.088 log units (1.6 dB) every 1.1 cm (Figure 6). The observer is given the first panel at a distance of 50 cm, completely covered with a sheet of paper except for the upper portion, where the sine-wave bars are not detectable. The direction of presentation (from minimum to maximum contrast) is therefore opposite to that adopted in the Pelli-Robson chart (from maximum to minimum contrast letters). The plate is then slowly uncovered. The observer is asked to report the position of the sheet as soon as he can detect the bars. A scale on the side of the plate converts the position of the sheet into the threshold. Then, the second plate is presented and the examination is iterated for all the plates so to obtain a threshold per spatial frequency tested. The thresholds obtained at the different spatial frequencies allow plotting the CSF.
The procedure follows the method of limits for continuous stimulation (adjustment).
The 4AFC CS test
Vaegan and Halliday (35) proposed a 4-AFC response model to assess CS. Parts of Arden’s gratings are cut into a series of discs that differ in contrast and spatial frequency to be presented in four different orientations (horizontal, vertical, left, or right oblique). The same six frequencies of the Arden gratings are administered. The disk is presented with a certain orientation and the observer is forced to report the orientation of the grating. The threshold corresponds to the grating with the lowest contrast whose orientation is recognized at each spatial frequency.
The Cambridge low contrast gratings
The Cambridge low contrast gratings (36) administer square-wave stimuli to test CS within a range of eleven values, from 0.89 to 2.85 log contrast (i.e., from 13% to 0.11% contrast value), at a spatial frequency of 4 cycles/deg. The authors decided to test only the spatial frequency of 4 c/deg since they assumed that an alteration of any other frequencies always implies a deficit at 4 c/deg. Moreover, the peak in CS is observed at spatial frequencies between 3 and 6 c/deg (37): these are the spatial frequencies that are necessary to perform common visual tasks (38). A grating and a null (uniform) stimulus are presented at a distance of 6 meters; the observer is asked to indicate the target (the grating). The test is repeated four times at each level of contrast: In the original version, a score is obtained from the total number of detected gratings. The score is then converted into CS. The procedure uses a forced-choice response design (2AFC), method of constant stimuli (4 constant stimuli for each contrast value), detection task. Jones and colleagues found that the repeatability varies by about one-third of the total performance range, therefore they recommend caution in monitoring CS with this method (39).
The Sine Wave Contrast Test Vistech CS charts (Ginsburg’s gratings)
Ginsburg (40) developed a test based on the detection of sine-wave gratings vertically oriented or rotated by 15 degrees clockwise or counterclockwise. Five different spatial frequencies are administered (1.5, 3, 6, 12, and 18 c/deg) at 9 contrast levels (non-constant step size with an average value of 0.25 logarithmic units: Figure 7). There is a version for far distance (3 meters) and a version for near distance (40 centimeters).

The method of adjustment with manual contrast control
Another technique developed by Vaegan and Halliday (35) makes use of the method of adjustment and administers gratings with predefined spatial frequency. The contrast of each grating is progressively increased by the observer until the pattern is detected (the operator ensures during the examination that the speed at which the observer increases the contrast of the grating is constant and around 0.5 dB/sec). This method is quick and simple but is strictly dependent on the observer’s response criterion. According to the authors, decreasing series are not suitable since they are much more variable. In effect, gradual reduction of intensities leads the observer to adapt to the previous high contrast stimulation, resulting in an overestimation of the threshold (1). Another method of adjustment, called von Békésy tracking, administers ascending and descending series of contrast levels; after a predetermined number of reversals in the trend of the responses, detection threshold is estimated as the mean of the reversal points. However, repeatability appeared to be lower than for the classical method of adjustment with increasing series (41).
The Freiburg visual Acuity and Contrast Test (FrACT)
FrACT (42,43) assesses not only high-contrast visual acuity but also CS for a given angular dimension of the stimulus. As for the measurement of visual acuity, it is an 8-AFC response design, with the detection task focused on the orientation of Landolt’s “C”. Presentations are guided by the best PEST psychophysical procedure. Best PEST is a Bayesian procedure (like the MEEE method) than can quickly estimate the threshold, assuming that the slope of the psychometric function is known (31).
The Holladay Automated Contrast Sensitivity Testing System (HACSS)
The HACSS (44) administers concentric circular sine-wave stimuli with a spatial frequency of 1.5, 3, 6, 12, 18 c/deg. At each frequency, the stimulus is presented starting from a contrast level of 50% (viewing distance: 4 meters). The observer must report if he detects a concentric target (Figure 8) or if he sees a uniform circle. The operator presses a key corresponding to the patient’s response. The program includes a protocol for photopic vision (luminance: 85 cd·m-2) and one for mesopic vision (luminance: 3 cd·m-2) by applying a filter to the monitor.

Contrast is initially reduced by 0.3 log units for each correct answer, then by 0.1 log units near the threshold. In the case of a wrong response, the contrast is increased by 0.3 log units, which becomes 0.2 after the second wrong response. The threshold corresponds to the lowest contrast at which the subject detects two out of three stimuli. The threshold is computed as the mean of the two best results before the next incorrect answer. The CSF is obtained by repeating the procedure for each spatial frequency. During the exam, null stimuli are presented as false-positive controls and a reliability index is computed.
HACSS follows a simple up-down staircase procedure associated with a yes/no response model, but the step size is reduced at a certain point of the examination.
Which is the best way to measure CS?
It should be borne in mind that the estimation of CS, in terms of absolute values and shape of the CSF, depends on the response model employed and on the psychophysical technique (45). Alternative forced-choice (AFC) responses should be preferred over criterion-dependent methods (yes/no model) because they minimize the undesired effect of the subjective criterion, increasing reliability and repeatability (18,35). Within the framework of the signal detection theory, the subjective criterion varies depending on the degree of confidence of the subject in detecting the signal (46). Contrary to the yes/no response model, alternative-forced choice designs rule out this problem. Pelli and colleagues suggested using at least 4 or 5 alternatives to minimize the guessing rate and make CS assessment more accurate (29).
Which is the best stimulus to be administered is a debated topic. Letter charts measure recognition while gratings refer to a detection task (31,47): the two thresholds do not match and, as pointed out by Leguire, some clinical conditions could influence them to a different extent (47). The same author suggested that recognition of letters is influenced by their different legibility, even if Pelli and Regan considered this aspect not relevant (48,49). What is substantial is that the Pelli-Robson chart does not provide the shape of the CSF, but measures CS at a predetermined spatial frequency (that is a function of the adopted viewing distance). It follows that it is not suitable to detect high frequency (type 1) CS losses (47,50). Despite this shortcoming, letter charts are simple, quick and easy to be administered, and provide reliable results. Moreover, the Pelli-Robson chart does not suffer from ceiling and floor effects, and reliability and repeatability are better compared to gratings (29,51,52) and to the computerized test FrACT, that makes use of Landolt Cs (53).
Low-contrast optotypes (LCSLC) have a smaller step size and use a higher number of forced-choice alternatives compared to grating-based CS tests like the Vistech chart (0.10 vs. 0.25 log steps and 26 vs. 3 AFC, respectively), resulting in higher precision and reliability compared to the former (33,51). Their peculiarity is that they assess how visual acuity is affected by contrast, i.e., they measure the smallest letter that can be resolved at a predetermined contrast, but do not provide a direct estimate of CS.
Indeed, test-retest reliability and validity of grating tests are reported to be quite poor [in particular in the original version of the Vistech (51,52)], especially at the lower spatial frequencies. Despite they are suitable for screening purposes (34), this suggests caution in clinical use (52). It remains that gratings show a substantial advantage over letters and other optotypes, that is they plot the CSF: a valuable piece of information about the overall integrity of the visual system (4). Sinusoidal gratings should be preferred to square waves because of their luminance profile that is robust against defocus, optical aberrations, diffraction, or light scatter (1).
Regarding the psychophysical procedure, non-adaptive procedures like the method of limits with increasing (ascendent) stimuli provides better reliability than the method of adjustment, and its variant von Békési tracking (41). The method of constant stimuli, indeed, is the most simple way to compute the psychometric function of the observer (31) but is too time-consuming, i.e., not efficient, for the clinical purpose (54).
Adaptive strategies reduce examination time. Parametric procedures like best PEST (adopted by the FrACT) are accurate and less time-consuming than the nonparametric counterparts (31), while test-retest variability is quite similar (55). Of note, patients may have difficulty familiarizing themselves with parametric strategies, due to the fast convergence toward the threshold since the initial phase of the examination (55).
A final question arises about whether different visual pathways can be investigated via different techniques. As highlighted by an anonymous reviewer, it is worth recalling that all the tests discussed so far measure CS at an overall perceptual level. Even if some of them are focused on a specific range of spatial frequencies, none of them selectively target, i.e., isolate, the magnocellular or the parvocellular CSF; due to the wide overlapping of the P- and M-function, indeed, both are always involved in the CS estimates.
The critical fusion frequency (CFF)
The investigations reported up to this point addressed the problem from the perspective of spatial contrast, and analyzed CS as a function of the visual angle subtended by the target in stationary temporal conditions. A different strand of research focused on temporal contrast, that is to say on the sensitivity of the visual system as a function of the flicker frequency of a grating with fixed spatial frequency.
Temporal CS in the absence of a spatial component is defined as the sensitivity to luminance differences within the temporal domain, i.e., as a function of time. Likewise, the limit to temporal sensitivity of the visual system is reflected in the CFF that is the temporal frequency at which a flashing target is no longer perceivable as flickering.
This threshold depends on the neuronal limitation in encoding signals in the temporal domain: the neurons are unable to modulate tachistoscopic information beyond a certain temporal frequency: it follows that the flickering stimulus is perceived as stationary.
CFF is considered a good indicator of the integrity of visual temporal processing, abnormal in encephalopathies, ocular conditions such as multiple sclerosis with involvement of the optical pathways (56,57), macular degeneration (58), cataract (59), and glaucoma (60).
The amount of temporal modulation of the visual stimulus is the modulation amplitude, that is the oscillations of luminance over time. Modulation amplitude can be expressed as a modulation percentage (Figure 9).
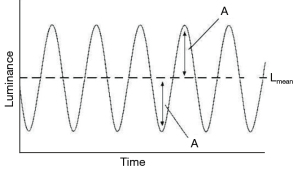
The performance of the visual system as a function of the temporal frequency and the modulation percentage is described by the temporal modulation transfer function (TMTF). TMTF implies a multidimensional analysis, like the CSF, and its shape is similar to the latter, with a central peak, a steep decay at high temporal frequencies, and a more gradual decrease at low temporal frequencies (Figure 10). According to this paradigm, the CFF measures the visual temporal resolution. It corresponds to the temporal frequency at which the flicker is no longer detected (i.e., the threshold) at a certain modulation percentage.
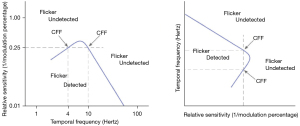
CFF increases with the logarithm of the size of the target: the greater the stimulus, the greater the CFF, thereby the easier the detection of the flicker (61). This trend is in agreement with the different retinal distribution of parvo- and magnocells: the magnocellular (or transient) system is more represented in the peripheral retina, therefore its activation grows as the target centered to the fovea is made larger. CFF varies also as a function of contrast, adaptation to light, aging, and, above all, as a function of stimulus intensity (62,63): in fact, CFF increases directly with the logarithm of the target’s luminance. The relationship is expressed by the Ferry-Porter law:
where I is the stimulus luminance, I0 is the threshold luminance, and k has a typical value of 10–30 Hz (64).
To date, it is not simple to establish which is the best paradigm for estimating the CFF. Eisen-Enosh and colleagues compared the method of limits associated with a yes/no response model, the method of constant stimuli, and the staircase both coupled to a 2AFC temporal paradigm. Test-retest reliability is satisfactory for all three methods, albeit slightly lower for the limits (65). Since the staircase is an adaptive procedure that combines good accuracy with a short execution time, it may be considered the most suitable.
Conclusions
The visual system relies on multiple channels to transmit information from the retina to the higher centers.
In the spatial domain, high contrast stimuli, used for measuring central visual acuity, recruit mainly the parvocellular pathway. As a consequence, estimating visual acuity may not yield a comprehensive overview of the functional status of the visual system: as a matter of fact, there are pathological conditions that affect preferentially the magnocellular system. In these cases, a decline in CS, both in the spatial and temporal domain, takes place in the face of a normal capacity to discriminate and identify fine details. The importance of measuring CS relies upon this basis, and is demonstrated by the considerable number of psychophysical procedures proposed so far for its assessment as a complement to visual acuity. It is likely that, among those reported in this paper, there is not a technique that should be preferred to the others, since each of them has its pros and cons, some are more suitable for screening purposes, others are more suitable for evaluating the effect of this function on specific tasks like, for example, reading.
Future directions in this field involve virtual reality-based tests (66) or the use of novel stimuli that make use of illusory motion to generate contrast, and whose effectiveness in measuring CS in patients with retinal vein occlusion and age-related macular degeneration has been recently reported (67,68). Finally, a method to measure CS based on real-time detection of the optokinetic response has been proposed and found effective in detecting the effect of defocus on CS in emmetropic subjects (69). The development of these new techniques will substantially contribute to imporving the early diagnosis and treatment of ophthalmic pathologies based on CS.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://aes.amegroups.com/article/view/10.21037/aes-22-26/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-22-26/coif). CA reports in the past 36 months, royalties paid for the development of the Tetra Analyzer, a device that contains a test for contrast sensitivity, and royalties due for 3 books and paid lectures on dyslexia and perception, where the principles of contrast sensitivity and its estimation are cited. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benjamin WJ. Borish’s clinical refraction. Butterworth Heinemann Elsevier, 1988.
- BLACKWELL HR. Contrast thresholds of the human eye. J Opt Soc Am 1946;36:624-43. [Crossref] [PubMed]
- Lesmes LA, Jeon ST, Lu ZL, et al. Bayesian adaptive estimation of threshold versus contrast external noise functions: the quick TvC method. Vision Res 2006;46:3160-76. [Crossref] [PubMed]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J Physiol 1968;197:551-66. [Crossref] [PubMed]
- Lee BB, Smith VC, Pokorny J, et al. Rod inputs to macaque ganglion cells. Vision Res 1997;37:2813-28. [Crossref] [PubMed]
- Purpura K, Kaplan E, Shapley RM. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc Natl Acad Sci U S A 1988;85:4534-7. [Crossref] [PubMed]
- Merigan WH, Maunsell JH. Macaque vision after magnocellular lateral geniculate lesions. Vis Neurosci 1990;5:347-52. [Crossref] [PubMed]
- Tolhurst DJ. Reaction times in the detection of gratings by human observers: a probabilistic mechanism. Vision Res 1975;15:1143-9. [Crossref] [PubMed]
- Legge GE. Sustained and transient mechanisms in human vision: temporal and spatial properties. Vision Res 1978;18:69-81. [Crossref] [PubMed]
- Maunsell JH, Nealey TA, DePriest DD. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J Neurosci 1990;10:3323-34. [Crossref] [PubMed]
- Schiller PH, Logothetis NK, Charles ER. Functions of the colour-opponent and broad-band channels of the visual system. Nature 1990;343:68-70. [Crossref] [PubMed]
- Schiller PH, Logothetis NK, Charles ER. Role of the colour-opponent and broad-band channels in vision. Vis Neurosci 1990;5:321-46. [Crossref] [PubMed]
- Merigan WH, Katz LM, Maunsell JH. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. J Neurosci 1991;11:994-1001. [Crossref] [PubMed]
- Merigan WH, Eskin TA. Spatio-temporal vision of macaques with severe loss of Pb retinal ganglion cells. Vision Res 1986;26:1751-61. [Crossref] [PubMed]
- Regan D, Neima D. Low-contrast letter charts as a test of visual function. Ophthalmology 1983;90:1192-200. [Crossref] [PubMed]
- Green DG, Campbell FW. Effects of focus on the visual response to a sinusoidally modulated spatial stimulus. J Opt Soc Am 1965;55:1154-7. [Crossref]
- Freeman RD, Thibos LN. Contrast sensitivity in humans with abnormal visual experience. J Physiol 1975;247:687-710. [Crossref] [PubMed]
- Higgins KE, Jaffe MJ, Caruso RC, et al. Spatial contrast sensitivity: effects of age, test-retest, and psychophysical method. J Opt Soc Am A 1988;5:2173-80. [Crossref] [PubMed]
- Schieber, F, Kline DW, Kline TJ, et al. Contrast sensitivity and the visual problems of older drivers (SAE Technical Paper No. 920613). Warrendale, PA: Society of Automotive Engineers, 1992.
- Regan D, Silver R, Murray TJ. Visual acuity and contrast sensitivity in multiple sclerosis--hidden visual loss: an auxiliary diagnostic test. Brain 1977;100:563-79. [Crossref] [PubMed]
- Regan D. The Charles F. Prentice Award Lecture 1990: specific tests and specific blindnesses: keys, locks, and parallel processing. Optom Vis Sci 1991;68:489-512. [Crossref] [PubMed]
- Storch RL, Bodis-Wollner I. Overview of contrast sensitivity and neuro-ophthalmic disease. In: Nadler MP, Miller D, Nadler DJ. editors. Glare and Contrast Sensitivity for Clinicians. New York: Springer-Verlag, 1990.
- Aleci C. Dyslexia: A Visual Approach. New York: Nova Science Publishers Inc., 2013.
- Lovegrove WJ, Bowling A, Badcock D, et al. Specific reading disability: differences in contrast sensitivity as a function of spatial frequency. Science 1980;210:439-40. [Crossref] [PubMed]
- Lovegrove W, Slaghuis W, Bowling A, et al. Spatial frequency processing and the prediction of reading ability: a preliminary investigation. Percept Psychophys 1986;40:440-4. [Crossref] [PubMed]
- Cornelissen P, Richardson A, Mason A, et al. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vision Res 1995;35:1483-94. [Crossref] [PubMed]
- Skottun BC. The magnocellular deficit theory of dyslexia: the evidence from contrast sensitivity. Vision Res 2000;40:111-27. [Crossref] [PubMed]
- Cobo-Lewis AB. An adaptive psychophysical method for subject classification. Percept Psychophys 1997;59:989-1003. [Crossref] [PubMed]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci 1988;2:187-99.
- Robson JG. Contrast sensitivity: one hundred years of clinical measurement. In: Shapley R, Lam DMK. editors. Contrast Sensitivity. Cambridge: MIT Press, 1993.
- Aleci C. Measuring the Soul-Psychophysics for non-Psychophysicists, EDP Sciences. France: Les Ulis, 2020.
- Haymes SA, Roberts KF, Cruess AF, et al. The letter contrast sensitivity test: clinical evaluation of a new design. Invest Ophthalmol Vis Sci 2006;47:2739-45. [Crossref] [PubMed]
- Balcer LJ, Baier ML, Pelak VS, et al. New low-contrast vision charts: reliability and test characteristics in patients with multiple sclerosis. Mult Scler 2000;6:163-71. [Crossref] [PubMed]
- Arden GB, Jacobson JJ. A simple grating test of contrast sensitivity. Preliminary results indicate value in screening for glaucoma. Invest Ophthalmol Vis Sci 1978;17:23-32. [PubMed]
- Vaegan, Halliday BL. A forced-choice test improves clinical contrast sensitivity testing. Br J Ophthalmol 1982;66:477-91. [Crossref] [PubMed]
- Wilkins AJ, Della Sala S, Somazzi L, et al. Age-related norms for the Cambridge low contrast gratings, including details concerning their design and use. Clin Vis Sci 1988;2:201-12.
- Ginsburg AP. Contrast sensitivity and functional vision. Int Ophthalmol Clin 2003;43:5-15. [Crossref] [PubMed]
- Richman J, Spaeth GL, Wirostko B. Contrast sensitivity basics and a critique of currently available tests. J Cataract Refract Surg 2013;39:1100-6. [Crossref] [PubMed]
- Jones HS, Moseley MJ, Thompson JR. Reliability of the Cambridge Low Contrast Gratings. Ophthalmic Physiol Opt 1994;14:287-9. [Crossref] [PubMed]
- Ginsburg AP. A new contrast sensitivity vision test chart. Am J Optom Physiol Opt 1984;61:403-7. [Crossref] [PubMed]
- Ginsburg AP, Cannon MW. Comparison of three methods for rapid determination of threshold contrast sensitivity. Invest Ophthalmol Vis Sci 1983;24:798-802. [PubMed]
- Bach M. The Freiburg Vision Test. Automated determination of visual acuity. Ophthalmologe 1995;92:174-8. [PubMed]
- Bach M. The Freiburg Visual acuity test-automatic measurement of visual acuity. Optom Vis Sci 1996;73:49-53. [Crossref] [PubMed]
- van Gaalen KW, Jansonius NM, Koopmans SA, et al. Relationship between contrast sensitivity and spherical aberration. Comparison of 7 contrast sensitivity tests with natural and artificial pupils in healthy eyes. J Cataract Refract Surg 2009;35:47-56. [Crossref] [PubMed]
- Woods LR, Thomson WD. A comparison of psychometric methods for measuring the contrast sensitivity of experienced observers. Clin Vis Sci 1993;8:401-15.
- Green DA, Swets JA. Signal detection theory and psychophysics. Oxford: John Wiley, 1966.
- Leguire LE. Do letter charts measure contrast sensitivity? Clin Vision Sci 1991;6:391-400.
- Regan D. Do letter charts measure contrast sensitivity? Clin Vis Sci 1991;6:401.
- Pelli DG, Robson JG. Are letters better than gratings? Clin Vis Sci 1991;6:409.
- Elliott DB, Situ P. Visual acuity versus letter contrast sensitivity in early cataract. Vision Res 1998;38:2047-52. [Crossref] [PubMed]
- Elliott DB, Bullimore MA. Assessing the reliability, discriminative ability, and validity of disability glare tests. Invest Ophthalmol Vis Sci 1993;34:108-19. [PubMed]
- Rubin GS. Reliability and sensitivity of clinical contrast sensitivity tests. Clin Vis Sci 1988;2:169-77.
- Bühren J, Terzi E, Bach M, et al. Measuring contrast sensitivity under different lighting conditions: comparison of three tests. Optom Vis Sci 2006;83:290-8. [Crossref] [PubMed]
- Watson AB, Fitzhugh A. The method of constant stimuli is inefficient. Percept Psychophys 1990;47:87-91. [Crossref] [PubMed]
- Madigan R, Williams D. Maximum-likelihood psychometric functions in two-alternative forced-choice: evaluation and recommendations. Percept Psychophys 1987;42:240-9. [Crossref] [PubMed]
- Kircheis G, Wettstein M, Timmermann L, et al. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology 2002;35:357-66. [Crossref] [PubMed]
- Salmi T. Critical flicker frequencies in MS patients with normal or abnormal pattern VEP. Acta Neurol Scand 1985;71:354-8. [Crossref] [PubMed]
- Phipps JA, Dang TM, Vingrys AJ, et al. Flicker perimetry losses in age-related macular degeneration. Invest Ophthalmol Vis Sci 2004;45:3355-60. [Crossref] [PubMed]
- Shankar H, Pesudovs K. Critical flicker fusion test of potential vision. J Cataract Refract Surg 2007;33:232-9. [Crossref] [PubMed]
- Tyler CW. Specific deficits of flicker sensitivity in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 1981;20:204-12. [PubMed]
- Granit R, Harper P. Comparative studies on the peripheral and central retina. II. Synaptic reactions in the eye. Am J Physiol 1930;95:211-28. [Crossref]
- Tyler CW. Analysis of visual modulation sensitivity. II. Peripheral retina and the role of photoreceptor dimensions. J Opt Soc Am A 1985;2:393-8. [Crossref] [PubMed]
- Tyler CW. Analysis of visual modulation sensitivity. III. Meridional variations in peripheral flicker sensitivity. J Opt Soc Am A 1987;4:1612-9. [Crossref] [PubMed]
- Tyler CW, Hamer RD. Eccentricity and the Ferry-Porter law. J Opt Soc Am A Opt Image Sci Vis 1993;10:2084-7. [Crossref] [PubMed]
- Eisen-Enosh A, Farah N, Burgansky-Eliash Z, et al. Evaluation of Critical Flicker-Fusion Frequency Measurement Methods for the Investigation of Visual Temporal Resolution. Sci Rep 2017;7:15621. [Crossref] [PubMed]
- Vivas-Mateos G, Boswell S, Livingstone IAT, et al. Screen and Virtual Reality-Based Testing of Contrast Sensitivity. Annu Int Conf IEEE Eng Med Biol Soc 2020;2020:6054-7. [Crossref] [PubMed]
- Mishra S, Maganti N, Squires N, et al. Contrast Sensitivity Testing in Retinal Vein Occlusion Using a Novel Stimulus. Transl Vis Sci Technol 2020;9:29. [Crossref] [PubMed]
- Maganti N, Squires N, Mishra S, et al. Contrast Sensitivity Testing in Age-Related Macular Degeneration Using Motion Diamond Stimulus. Clin Ophthalmol 2022;16:507-15. [Crossref] [PubMed]
- Essig P, Sauer Y, Wahl S. Contrast Sensitivity Testing in Healthy and Blurred Vision Conditions Using a Novel Optokinetic Nystagmus Live-Detection Method. Transl Vis Sci Technol 2021;10:12. [Crossref] [PubMed]
Cite this article as: Rosa C, Aleci C. Psychophysics in the ophthalmological practice—II. Contrast sensitivity. Ann Eye Sci 2022;7:35.




