
Changes in corneal curvature and aberrations after cataract surgery
Introduction
The refractive power of the cornea accounts for two-third of the total refractive power (1). In recent years, with the development of refractive cataract surgery, an increasing number of cataract surgeons have paid attention to accurately evaluating the corneal shape and refractive properties of patients. It is of great significance for reducing surgically induced astigmatism (SIA), accurately implanting suitable multifocal or toric intraocular lenses (IOLs) and eventually elevating the visual quality of patients after cataract surgery (2-4).
SIA and corneal high-order aberrations (HOAs) are the two main causes of poor visual quality after cataract surgery (2,5). Previously, SIA was evaluated mainly based on changes in anterior corneal curvature, which reflected the astigmatism of the anterior corneal surface without considering the curvature of the posterior corneal surface (6,7). The role of posterior corneal curvature in total corneal astigmatism remains unclear. Previous studies have found that corneal HOAs account for most whole-eye aberrations, which is one of the important factors affecting the visual quality of patients after cataract surgery (8,9). However, changes in the parameters of corneal HOAs after cataract surgery and their effects on and in relationships with changes in corneal curvature have not been reported.
In this study, we investigated the changes in and effects of anterior, posterior, total corneal curvature and corneal HOAs after 2.2 mm temporal microincision coaxial phacoemulsification to identify ideal methods to achieve the best visual quality in patients after cataract surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://aes.amegroups.com/article/view/10.21037/aes-22-4/rc).
Methods
Subjects
According to the Declaration of Helsinki, this prospective study was conducted at the Zhongshan Ophthalmic Center of Sun Yat-sen University from April 1 to August 31, 2019 for consecutive patients undergoing phacoemulsification with IOL implantation. Only right eyes were included for patients undergoing surgery in both eyes. All patients provided written informed consent after a thorough explanation of the study process, risks and possible complications. This study was performed in compliance with the principles of the Declaration of Helsinki (as revised in 2013) and approved by the ethical committee of Zhongshan Ophthalmic Center (No. 2019KYPJ091).
Age-related cataract patients with a lens opacification classification system III (LOCS III) grade ≥3.0 aged 55–90 years were included. The exclusion criteria were as follows: a history of eye surgery or ocular diseases, inability of the pupil to dilate to ≥6 mm, risk of posterior capsular rupture, lens dislocation, and LOCS III nuclear opalescence grade >6.0. Diabetic patients were also excluded from the study as the blood glucose levels associated with the disease can alter the biomechanical properties of the cornea.
Surgical technique
All surgeries were performed by the same ophthalmologist (YL) following a standardized procedure for microincision coaxial phacoemulsification with IOL implantation. One drop each of topical tropicamide 0.5% (Shenyang Xingqi, Shenyang, China) and promecaine hydrochloride 0.5% (Novartis, Antwerp, Belgium) was administered to the surgical eye every 5 minutes for a total of three times before surgery. A 2.2-mm dual-bevel keratome (Alcon Labs, Clear Cut Intrepid SB, Fort Worth, TX, USA) was used to make a transparent temporal two-plane corneal incision. Injection of an ophthalmic viscoelastic device consisting of medical sodium hyaluronate gel (Hangzhou Singclean Medical Products Co., Ltd, Hangzhou, China) was used to maintain the stability of the anterior chamber. A 26G capsulotomy needle was used to create a continuous circular capsulorhexis of 5.5–6.0 mm in diameter. Hydrodissection was done through the main incision. The Centurion Vision System (Alcon Labs) was used to perform phacoemulsification, including nucleus chopping with a 0.9-mm Ultra-sound tip, a CENTURION® OZil® handpiece (Alcon Labs) and a straight-headed coaxial tip for irrigation-aspiration (I/A). Torsional phacoemulsification was set between 60% and 100%, suction velocity was 33–35 mL/min, and negative pressure was maintained in the range 330–350 mmHg during phacoemulsification. A monofocal IOL (SN60WF) was implanted. The parameters included the operation time, cumulative dissipated energy (CDE), ultrasound time, I/A time and total fluid volume.
Patient assessment
The patients were examined before the operation and at 1 day (POD1), 7 days (POD7) and 3 months (POM3) postoperatively. Refraction, intraocular pressure, slit lamp and fundus examinations and anterior segment optical coherence tomography (AS-OCT) (Tomey SS-1000, Aichi, Japan) were used to evaluate the total, anterior and posterior corneal astigmatism and direction for its good repeatability and precision (10-12). iTrace (Tracey Technologies, Houston, TX, USA) was used to assess corneal HOAs with the pupil diameter at 5.0 mm.
The nuclear opalescence of the lens was graded by the LOCS III (13). The corrected distance visual acuity (CDVA) was measured on decimal charts, and the decimal visual acuity was converted to the logarithm of the minimum angle of resolution (logMAR) scale for statistical analysis. Corneal astigmatism was defined as with-the-rule astigmatism (WTR), which is a steep corneal curvature between 60° and 120°, and against-the-rule astigmatism (ATR), which is corneal curvature both between 0° and 30° and between 150° and 180°; others were defined as oblique astigmatism. Corneal astigmatism was analyzed by the method of power vectors, in which corneal power (Ksteep @θ1 and Kflat @θ2) is converted into the Jackson cross-cylinder with axes at 180° and 90° (J0) and the Jackson cross-cylinder with axes at 45° and 135° (J45) (14). The SIA was calculated at each postoperative visit using polar value analysis (15). The parameters of corneal HOAs were recorded by Zernike coefficient data as follows: 3rd-order trefoil, Z (3, −3) (vertical trefoil) and Z (3, +3) (oblique trefoil); coma Z, (3, −1) (vertical coma) and Z (3, +1) (horizontal coma); and 4th-order spherical aberration, Z (4, 0).
Statistical analysis
The sample size was calculated based on the change of corneal HOAs at 1 day after surgery (16). A sample size of 52 achieving for 80% power was required to detect a mean change of 0.08 with standard deviation of 0.20 using paired t-test with a two-sided significance level (alpha) of 0.05. Anticipating 10% loss to follow-up at 3 months, we aimed to enrolled 58 participants. PASS 16.0 (NCSS, LLC, Kaysville, UT, USA) was used to calculate the sample size. SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used for the data analyses. All missing data were imputed by creating 20 copies of the data, in which missing values were imputed using multiple imputation in Stata (17). The differences between preoperative and postoperative data were analyzed by mixed-model analysis of variance for repeated measures. Subsequent mean comparisons were made by the Bonferroni method. The paired t-tests were used to compare the preoperative data and postoperative data at each visit. P values <0.05 were considered to be statistically significant.
Results
A total of 61 patients (61 eyes) were included (Figure 1). The demographic characteristics of these patients are summarized in Table 1. The types of corneal astigmatism and the distribution of astigmatism of the anterior, posterior and total corneal surfaces before cataract surgery are shown in Table 2 and Figure 2. As shown in Figure 3, for the anterior corneal surface, the ratio of WTR was dramatically increased at 3 months postoperatively (39.3%) compared with preoperatively (21.3%). Conversely, the ratio of ATR at 3 months postoperatively (32.8%) was decreased compared with preoperatively (49.2%). This tendency was also observed for the total corneal surface. These changes induced similar ratios for WTR, ATR and oblique astigmatism for the anterior and total corneal surfaces at 3 months postoperatively. Changes in the astigmatism type of the posterior corneal surface were less than those of the anterior and total corneal surfaces. WTR astigmatism was the main type of astigmatism of the posterior corneal surface (96.7%) before surgery, and while this proportion decreased slightly at 3 months after surgery (85.2%), it was still the main type of astigmatism. Notably, the proportion of posterior oblique astigmatism increased dramatically at 3 months postoperatively (11.5%) compared with preoperatively (1.6%).
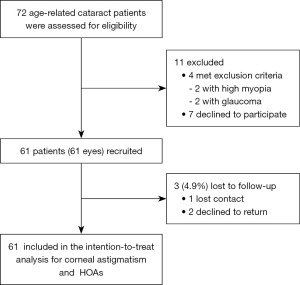
Table 1
| Characteristics | Finding |
|---|---|
| Age (mean ± SD) | 72.2±7.87 |
| Sex, n (%) | |
| Male | 30 (49.2) |
| Female | 31 (50.8) |
| Eye (right/left) | 31/30 |
| Lens nuclear opalescence grade (LOCS III, mean ± SD) | 3.33±0.820 |
| UCDVA, logMAR (mean ± SD) | 0.961±0.461 |
| CDVA, logMAR (mean ± SD) | 0.819±0.492 |
LOCS III, lens opacification classification system III; UCDVA, uncorrected distance visual acuity; logMAR, logarithm of the minimum angle of resolution; CDVA, corrected distance visual acuity.
Table 2
| Astigmatism | Preoperative, n (%) |
|---|---|
| Anterior cornea | |
| With the rule | 13 (21.3) |
| Against the rule | 30 (49.2) |
| Oblique | 18 (29.5) |
| Posterior cornea | |
| With the rule | 59 (96.7) |
| Against the rule | 1 (1.6) |
| Oblique | 1 (1.6) |
| Total cornea | |
| With the rule | 8 (13.1) |
| Against the rule | 38 (62.3) |
| Oblique | 15 (24.6) |

The mean J0 and J45 values of anterior, posterior and total corneal curvature obtained by AS-OCT showed no statistically significant differences between preoperative data and any postoperative follow-up data in the mixed-model analysis (Table 3). No statistically significant differences between preoperative and 3-month postoperative data were found using paired t-tests (Table 4). SIA occurred on the anterior, posterior and total corneal surfaces, as shown in Table 5, with no statistically significant differences at any postoperative follow-up (Table 5).
Table 3
| Variable | Preop | POD1 | POD7 | POM3 | P | |
|---|---|---|---|---|---|---|
| Anterior curvature (mean ± SD) | ||||||
| J0 | −0.035±0.373 | −0.002±0.450 | −0.050±0.382 | −0.006±0.372 | 0.899 | |
| J45 | −0.063±0.367 | −0.032±0.498 | −0.036±0.398 | 0.028±0.398 | 0.654 | |
| Posterior curvature (mean ± SD) | ||||||
| J0 | 0.012±0.095 | −0.108±0.807 | −0.0150±0.093 | −0.008±0.094 | 0.308 | |
| J45 | −0.0002±0.072 | −0.001±0.168 | −0.011±0.083 | −0.017±0.097 | 0.732 | |
| Total curvature (mean ± SD) | ||||||
| J0 | 0.022±0.337 | 0.004±0.562 | 0.045±0.375 | 0.005±0.396 | 0.937 | |
| J45 | −0.024±0.386 | −0.006±0.406 | 0.020±0.367 | 0.016±0.327 | 0.892 | |
Preop, preoperative; POD1, postoperative day 1; POD7, postoperative day 7; POM3, postoperative month 3.
Table 4
| Variable | Preop | POM3 | P |
|---|---|---|---|
| Anterior curvature (mean ± SD) | |||
| J0 | −0.035±0.373 | −0.006±0.372 | 0.670 |
| J45 | −0.063±0.367 | 0.028±0.398 | 0.185 |
| Posterior curvature (mean ± SD) | |||
| J0 | 0.012±0.095 | −0.008±0.094 | 0.288 |
| J45 | −0.000±0.072 | −0.017±0.097 | 0.313 |
| Total curvature (mean ± SD) | |||
| J0 | 0.022±0.337 | 0.005±0.396 | 0.786 |
| J45 | −0.024±0.386 | 0.016±0.327 | 0.510 |
Preop, preoperative; POM3, postoperative month 3.
Table 5
| Variable | POD1 | POD7 | POM3 | P |
|---|---|---|---|---|
| Anterior SIA (mean ± SD) | 1.57±0.432 | 1.31±0.317 | 1.07±0.235 | 0.261 |
| Posterior SIA (mean ± SD) | 0.099±0.019 | 0.061±0.008 | 0.070±0.011 | 0.104 |
| Total SIA (mean ± SD) | 1.35±0.354 | 1.01±0.156 | 0.866±0.151 | 0.225 |
SIA, surgically induced astigmatism; POD1, postoperative day 1; POD7, postoperative day 7; POM3, postoperative month 3.
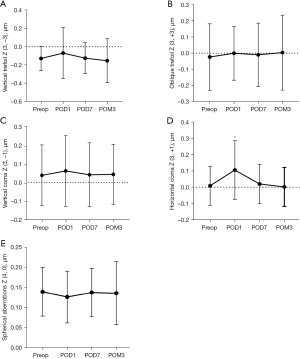
The time-dependent changes in corneal aberrations of the total corneal surface are shown in Figure 4. On the day after surgery, there was a significant increase in corneal 3rd-order horizontal coma (difference, −0.096; 95% CI: −0.177 to −0.015; P=0.012), which then gradually returned to baseline values at 3 months postoperatively (Figure 4A,4D). However, no significant changes in 3rd-order oblique trefoil, vertical coma or 4th-order spherical aberrations were observed after surgery (Figure 4B,4C,4E).
Discussion
Cataract surgery-induced corneal astigmatism consists of changes in anterior and posterior corneal curvature (18,19). Previously, astigmatism of the posterior surface was not considered for calculating the total corneal astigmatism because of the lack of equipment for exact measurement of the posterior surface (7,20). However, a recent study showed that if the total corneal astigmatism is calculated based only on the anterior surface and neglecting the posterior surface, the magnitude of astigmatism will be overestimated in WTR and underestimated in ATR (6,21). Moreover, Koch DD and Cheng et al. demonstrated that the omission of SIA of the posterior cornea may have a significantly detrimental impact on some patients after cataract surgery (19,21).
In this study, we explored changes in anterior, posterior and total corneal curvature after 2.2 mm temporal two-plane corneal incision phacoemulsification with IOL implantation for its common incision size in clinic (22), in which subjects mainly showed WTR astigmatism of the posterior corneal surface preoperatively. SIA, J0 and J45 of the anterior, posterior and total corneal surfaces did not show any statistically significant differences at any time point. These results indicate that 2.2 mm temporal two-plane corneal incision phacoemulsification was an ideal and stable incision for the tiny change in corneal shape. Changes in the astigmatism type of the posterior corneal surface were significantly less than those of the anterior and total corneal surfaces. These differences might be due to the diverse astigmatism types of the anterior and total corneal surfaces. Moreover, our results show that the magnitude of total corneal SIA, if based only on the anterior corneal surface, would be overestimated at any postoperative time point. It is suggested that posterior corneal SIA is important for the exact evaluation of vision quality after cataract surgery.
Some cataract patients achieved good uncorrected or corrected distance vision after surgery (Table S1), however, they complained of visual disturbances, including starbursts, halos and glare, which were induced by ocular HOAs generated by the cornea, intraocular lens, or other intraocular optical structures (9,23). It is worth noting that 80% of ocular aberrations occurred on the corneal surface (24). In addition, previous studies have found that patients treated with cataract surgery have larger corneal aberrations than those not treated with cataract surgery, and changes in corneal HOAs after surgery should not be ignored (16,24,25). In our study, we observed changes in corneal HOAs, including 3rd-order trefoil and coma and the 4th-order spherical aberrations that constitute the majority of HOAs after microincision coaxial phacoemulsification (26-28). Interestingly, the peak changes in horizontal coma among corneal HOAs occurred on POD1 and gradually returned to baseline on POD7. Other parameters of corneal HOAs did not change significantly at any postoperative time point compared with preoperatively. Horizontal coma represents the effect of horizontal incisions (8). Our results show that horizontal coma increased significantly at POD1, which implied that the CDVA was good, however, visual complaints were still presented by some patients after cataract surgery, especially at POD1.
The limitations of this study include the small sample size, and there was no analysis on changes in corneal curvature in patients of different ages after cataract surgery, which will be part of our work in the future. Moreover, we will use a new instrument such as Pentacam to investigate changes in anterior, posterior and total corneal HOAs after cataract surgery and the relationships among changes in anterior, posterior, and total corneal curvature and HOAs for the iTrace used in this study only detecting the total corneal HOAs.
Conclusions
We demonstrated that there were no significant changes in anterior, posterior or total corneal curvature after 2.2 mm temporal microincision coaxial phacoemulsification and that the corneal HOAs did not change significantly except for an increase in horizontal coma at POD1, which indicated poor visual quality at POD1 in some cataract patients which have good uncorrected or corrected distance vision.
Acknowledgments
We thank Zhenyu Wang and Ling Jin for the help of data analysis of the manuscript.
Funding: This study was supported by the Construction Project of High-Level Hospitals in Guangdong Province (No. 303020102), the National Natural Science Foundation of China (Nos. 81900815; 81873675) and the Fundamental Research Funds of the State Key Laboratory of Ophthalmology (No. 2019QN06).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aes.amegroups.com/article/view/10.21037/aes-22-4/rc
Data Sharing Statement: Available at https://aes.amegroups.com/article/view/10.21037/aes-22-4/dss
Peer Review File: Available at https://aes.amegroups.com/article/view/10.21037/aes-22-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-22-4/coif). YL serves as the unpaid Editor-in-Chief of Annals of Eye Science. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients provided written informed consent after a thorough explanation of the study process, risks and possible complications. This study was performed in compliance with the principles of the Declaration of Helsinki (as revised in 2013) and approved by the ethical committee of Zhongshan Ophthalmic Center (No. 2019KYPJ091).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Namba H, Sugano A, Nishi K, et al. Age-related variations in corneal geometry and their association with astigmatism: The Yamagata Study (Funagata). Medicine (Baltimore) 2018;97:e12894. [Crossref] [PubMed]
- Hoffmann PC, Abraham M, Hirnschall N, et al. Prediction of residual astigmatism after cataract surgery using swept source fourier domain optical coherence tomography. Curr Eye Res 2014;39:1178-86. [Crossref] [PubMed]
- Hayashi K, Sato T, Yoshida M, et al. Corneal shape changes of the total and posterior cornea after temporal versus nasal clear corneal incision cataract surgery. Br J Ophthalmol 2019;103:181-5. [Crossref] [PubMed]
- Hayashi K, Yoshida M, Hirata A, et al. Changes in shape and astigmatism of total, anterior, and posterior cornea after long versus short clear corneal incision cataract surgery. J Cataract Refract Surg 2018;44:39-49. [Crossref] [PubMed]
- Koç M, İlhan Ç, Koban Y, et al. Effect of corneal biomechanical properties on surgically-induced astigmatism and higher-order aberrations after cataract surgery. Arq Bras Oftalmol 2016;79:380-3. [Crossref] [PubMed]
- Koch DD, Ali SF, Weikert MP, et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg 2012;38:2080-7. [Crossref] [PubMed]
- Nemeth G, Berta A, Lipecz A, et al. Evaluation of posterior astigmatism measured with Scheimpflug imaging. Cornea 2014;33:1214-8. [Crossref] [PubMed]
- Song IS, Park JH, Park JH, et al. Corneal coma and trefoil changes associated with incision location in cataract surgery. J Cataract Refract Surg 2015;41:2145-51. [Crossref] [PubMed]
- Atchison DA, Suheimat M, Mathur A, et al. Anterior Corneal, Posterior Corneal, and Lenticular Contributions to Ocular Aberrations. Invest Ophthalmol Vis Sci 2016;57:5263-70. [Crossref] [PubMed]
- Wang Q, Chen M, Ning R, et al. The Precision of a New Anterior Segment Optical Coherence Tomographer and Its Comparison With a Swept-Source OCT-Based Optical Biometer in Patients With Cataract. J Refract Surg 2021;37:616-22. [Crossref] [PubMed]
- Jin GM, Xiao B, Zhou YJ, et al. Agreement of corneal curvature and central corneal thickness obtained from a swept-source OCT and Pentacam in ectopia lentis patients. Int J Ophthalmol 2020;13:1244-9. [Crossref] [PubMed]
- Schiano-Lomoriello D, Bono V, Abicca I, et al. Repeatability of anterior segment measurements by optical coherence tomography combined with Placido disk corneal topography in eyes with keratoconus. Sci Rep 2020;10:1124. [Crossref] [PubMed]
- Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993;111:831-6. [Crossref] [PubMed]
- Thibos LN, Horner D. Power vector analysis of the optical outcome of refractive surgery. J Cataract Refract Surg 2001;27:80-5. [Crossref] [PubMed]
- Naeser K, Hjortdal J. Polar value analysis of refractive data. J Cataract Refract Surg 2001;27:86-94. [Crossref] [PubMed]
- Ye H, Zhang K, Yang J, et al. Changes of corneal higher-order aberrations after cataract surgery. Optom Vis Sci 2014;91:1244-50. [Crossref] [PubMed]
- Royston P. Multiple imputation of missing values. The Stata Journal 2004;4:227-41. [Crossref]
- Nemeth G, Berta A, Szalai E, et al. Analysis of surgically induced astigmatism on the posterior surface of the cornea. J Refract Surg 2014;30:604-8. [Crossref] [PubMed]
- Cheng LS, Tsai CY, Tsai RJ, et al. Estimation accuracy of surgically induced astigmatism on the cornea when neglecting the posterior corneal surface measurement. Acta Ophthalmol 2011;89:417-22. [Crossref] [PubMed]
- Li C, Zhang J, Yin X, et al. Distribution and related factors of corneal regularity and posterior corneal astigmatism in cataract patients. Clin Ophthalmol 2019;13:1341-52. [Crossref] [PubMed]
- Koch DD, Jenkins RB, Weikert MP, et al. Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J Cataract Refract Surg 2013;39:1803-9. [Crossref] [PubMed]
- Luo L, Lin H, He M, et al. Clinical evaluation of three incision size-dependent phacoemulsification systems. Am J Ophthalmol 2012;153:831-9.e2. [Crossref] [PubMed]
- Hamam H. A new measure for optical performance. Optom Vis Sci 2003;80:175-84. [Crossref] [PubMed]
- He Q, Huang J, Xu Y, et al. Changes in total, anterior, and posterior corneal surface higher-order aberrations after 1.8 mm incision and 2.8 mm incision cataract surgery. J Cataract Refract Surg 2019;45:1135-47. [Crossref] [PubMed]
- von Sonnleithner C, Pilger D, Homburg D, et al. Corneal higher-order aberrations after phacoemulsification: a comparison of 3 different incision sizes. Eur J Ophthalmol 2017;27:402-6. [Crossref] [PubMed]
- Nanavaty MA, Spalton DJ, Marshall J. Effect of intraocular lens asphericity on vertical coma aberration. J Cataract Refract Surg 2010;36:215-21. [Crossref] [PubMed]
- López-Gil N, Rucker FJ, Stark LR, et al. Effect of third-order aberrations on dynamic accommodation. Vision Res 2007;47:755-65. [Crossref] [PubMed]
- Thibos LN, Hong X, Bradley A, et al. Statistical variation of aberration structure and image quality in a normal population of healthy eyes. J Opt Soc Am A Opt Image Sci Vis 2002;19:2329-48. [Crossref] [PubMed]
Cite this article as: Dai Y, Ruan X, Wang W, Chen X, Jin G, Wang L, Gu X, Qu B, Liu J, Tan X, Zhang E, Fu J, Luo L, Liu Z, Liu Y. Changes in corneal curvature and aberrations after cataract surgery. Ann Eye Sci 2022;7:25.


