
A revisit to staining reagents for neuronal tissues
Introduction
Building contrast of tissue within the field of histology was key to advancing scientific discoveries. The process of Gram staining for the distinction between bacteria based on the varying amounts of peptidoglycan within the cell wall proved to be revolutionary in identifying bacterial infections as well as the creation of the Nissl stain which provided the first opportunity for the visualization of the soma of the neuron. These small molecule staining reagents were the foundation for these discoveries, as well as countless others before the advancement in tissue visualization processes. Small molecule staining reagents have been used widely in various disciplinaries of scientific research, highlighting the importance of these methods. Particularly, in the field of both neuroscience and ophthalmology, there has been progressive discoveries in research due to the use of these staining techniques. However, these small molecule staining reagents are not limited to neurology and ophthalmology, for their use has been revolutionary in other fields as well such as microbiology and pathology.
Clinical medicine constantly experiences a threat of antimicrobial resistance. Gram negative bacteria are responsible for most acquired infections. Hans Christian Gram’s method of staining using a crystal violet-iodine complex with a saffron counterstain (1) discovered in 1884 enabled distinguishing Gram-negative from harmless Gram-positive bacteria. It is one subset of these small molecule staining reagents that have revolutionized medicine, towards fighting against antibiotic resistance and highlights the importance of small molecule staining reagents in the clinical setting.
Identifying the pathways underlying ocular, neuropsychiatric and neurodegenerative diseases such as several retinal disorders, schizophrenia and Alzheimer’s disease has been advanced by the creation of these small molecule staining techniques. Alzheimer’s disease is characterized by neuron degeneration results in diminished memory, and ultimately the loss of brain function. The use of the Golgi stain showed that the first cells to degenerate in Alzheimer’s disease are Cajal-Retzius neurons of the molecular layer of the cortex in both brain hemispheres. The disruptions of micro columnar cell arrangements led to investigation of synapse misfunction and was found to correlate with brain dysfunction (2). The tissue contrast produced by the Golgi stain showed extensive alteration of dendrites such as distortion of spines, numerous giant spines and substantial decrease of spine density in the visual cortex (2) within the brain of the Alzheimer’s patients. The small molecule staining reagents were a key contributor to the advancement in research in the fields of microbiology, neurology and pathology. However, vision science has proved promising leaps in research with the help of these small molecule staining reagents. Through hematoxylin and eosin (H&E) Staining, researchers have evaluated effects of ocular neovascularization (3). However, one of the frontiers of vision science is to understand how to regenerate neurons and reconnect them (4). This is important for one of the critical problems of ophthalmology today (4,5). That is restoration of vision in people who have lost the same due to traumatic injuries or due to diseases such as glaucoma or Leber hereditary optic neuropathy (5,6). Long distance regeneration of optic nerve neurons has been shown to be possible, though re-innervation and functional restoration still elude us. The detection of regeneration and re-innervation is key to establishing them. The detection of regeneration due to different intervention strategies including pharmacologically induced regeneration has been one of the critical problems of neuroscience.
Historic usage of small molecules as staining reagents
In early the 1700s, the neuronal control of bodily functions and involvement of peripheral neurons in control of muscle became increasing clear. Injuries leading to severance of nerves and loss of functions were well recognized by 1750 and there were consideration of repair and regeneration of nerves (7,8). Early studies of nerve degeneration mostly due to injuries and their potential regeneration suffered from lack of contrast. Early description of staining reagents found are the ones that attempted to develop contrast between nerve and the innervated tissues. Building contrast within samples is key when tracking regenerating and degenerating tissues. Staining reagents were used to target and track patterns and organization within tissue samples and enhance visibility (9). Before the advent of current microscopy and immunostaining methods, readily available and naturally occurring substances were used to target molecules within organelles. With the advent of antibodies against proteins (and other biomolecules) immunostaining has now taken central stage. Staining with small molecules (molecular weight less than 3,000 Daltons, usually in the range of 100–1,000 Daltons) is characterized by the use of naturally occurring substances such as dyes, inorganic or chemically synthesized small molecules to target organelles or abundant reactive molecules within a tissue sample. In this review such reagents have been grouped based on the region of the nervous system that they have been historically targeted or used: the central nervous system (CNS), the peripheral nervous system (PNS) or for both.
Staining reagents became widely used throughout the scientific community beginning in the late 1800s (Figure 1). Although such reagents have been used for the detection of organization across a multitude of tissues, nerve-related research greatly benefited from the advent of staining reagents. As scientific inquiry grew, the creation of new staining methods emerged in scientific literature. However, we found a lack of organized description or review small molecule reagents that is used for staining neuronal tissues, together with their chemical identities. We present here a comprehensive list of neuronal staining reagents based on their composition, optimal staining condition, use for given neuronal tissue and, where possible, historic usage, as well as future implications of these small molecule staining reagents.
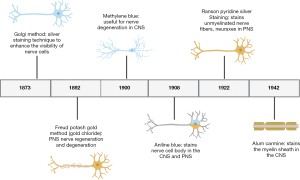
Utility of reagents for probing parts of CNS
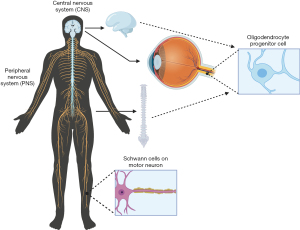
Within the CNS, composed of the brain and spinal cord within the body (Figure 2), degradation in parts of these neural systems or neurons possess a large threat to normal function. Historically usage of staining reagents helped to enhance the contrast and visibility of neuronal tissue and locate regions of nerve degeneration or regeneration due to interventional treatments. These small molecule staining reagents were generally used for tissues (and seldom for cells). The different cells (astrocytes, oligodendrocytes and microglial cells) and neurons within the CNS tissue reacted differently to different active ingredients of a specific stain. A large majority of these small molecules target and bind to myelin within the CNS. Myelin serves as an important insulator within the CNS that helps travel of neural impulses, accounting for the fast and highly selective nature of transmission of neural impulses. During nerve degeneration due to an injury or intrinsic disorder, the axon and surrounding glial cells begin to lose activity hindering nerve function overall in the affected region. Early staining reagents made it possible to track degeneration and/or recognize neural patterns throughout the brain and spinal cord during the phases of degeneration and that of regeneration against an intervention or pharmacological treatment.
CNS specific small molecule staining reagents that historically emerged in the scientific literature include saffron, thionin, methylene blue, carmine, Böhmer’s hematoxylin, Mallory’s phosphotungstic acid hematoxylin, Orange G, Iron hematoxylin, Prussian blue, Mallory-Heidenhain azan, periodic-acid Schiff, Schiff’s plasma reagent, Bielschowsky, Bethe Method, Paraldehyde, Alcian Blue, and Perl’s Prussian blue (https://cdn.amegroups.cn/static/public/aes-21-31-1.xlsx).
Utility of reagents for probing parts of PNS
The PNS is composed of the neurons outside of the brain and spinal cord. PNS serves as the bridge between the CNS and the limbs, organs and skin by sending nerve impulses from the CNS to the peripheral parts of the body. Small molecule staining reagents in the PNS are able to stain both cell bodies and myelin; however, the main source of myelin in the PNS is produced by Schwann cells, forming the myelin sheath (Figure 2). Nerve degeneration and other patterns within the nervous system in the PNS can be detected through the use of PNS specific small molecule staining reagents which include Osmic Acid, Hematoxylin and Eosin (H&E staining), Masson’s trichome stain, Poncaeu-fuchsin, phosphotungstic hematoxylin, Toluidine blue, Picric Acid-Hematoxylin Method, Picrocarmine, Ammonium Carminate, Ranson Pyridine Silver Staining, Sudan black B, Baker’s acid hematein method, and Verhoeff’s Elastic Stain (https://cdn.amegroups.cn/static/public/aes-21-31-1.xlsx).
Reagents that stain both CNS and PNS
Several reagents lack preference to stain tissue components in the CNS or the PNS, but instead stain components within both. An example is the gold method, which stains intercellular junctions, motor endplates, neuronal cell processes, and myelinated axons. The Nissl staining method also is used to stain components within both the CNS and the PNS, such as: RNA, Nissl bodies of nerve cells or other ribosomal aggregates.
A brief history of development of staining reagents
Listed below are a few neural staining reagents that have been used and modified throughout scientific research. Most reagents have specific compositions and methods for staining the neural tissue. Several stains that became named reagents later in the scientific literature are often derived from previous ones with suitable modification of original methods. A prime example of a stain that has been modified on several occasions is hematoxylin. Hematoxylin is a basic staining reagent, and its use has developed since the late 1800s. Böhmer’s hematoxylin is a staining method composed of hematoxylin crystals, potassium alum, and other chemicals. Its first documented use was in 1865, on patients that died from a cerebrospinal-medullary meningitis epidemic (10). While conducting autopsies, sections of tissue from the subarachnoid and arachnoid pia, as well as the cerebral cortex were collected and stained using Böhmer’s hematoxylin. This preparation is both less diffuse and more bound to the nuclei compared to the latter preparations of hematoxylin alum (10). Böhmer prepared hematoxylin using Campeche wood extract instead of alcoholic hematoxylin solution. The specimens placed in solution exhibited a more vibrant violet color compared to the blue that was produced by the alcoholic hematoxylin solution. Böhmer also used glycerin in his staining method which helped preserve the specimens for a longer period because of stabilization of the stain and prevention from oxidization. Böhmer’s method for hardening of the specimens involved the use of chromic acid and double-chromic acid potash but this was due to personal preference and not because of quality (10). Böhmer’s hematoxylin was later used by Howell in 1892, with picric acid to stain the axis cylinders of peripheral nerves (9). Picric acid is a strong acid coagulant and functions by changing the charges on the sidechains of proteins, thus disrupting hydrogen and electrostatic bonding. Since hydrogen bonds and electrostatic forces are integral to protein structure and function, the disruption of these forces causes the protein degradation. The myelin sheath, composed of protein, is broken down by the picric acid exposing the axis cylinders, leaving them vulnerable to staining by Böhmer’s hematoxylin (11). Hematoxylin, the major component of Böhmer’s hematoxylin, was later used in conjunction with different chemicals to stain other kinds of nerves and their parts. Following Böhmer’ use of hematoxylin to stain nerves, other researchers continued to use and enhance this staining method. Frank Burr Mallory, developed several modifications to hematoxylin that continue to be in use today as stains for nerves (and other tissues). Mallory discovered the phosphomolybdic acid hematoxylin (PMAH). One of the first recorded uses of this method was in 1914 to stain axis cylinders in a manner similar way to Böhmer’s hematoxylin. The PMAH behaved more specifically as well as more expansively than Böhmer’s. PMAH stained discrete granules in the axis cylinders, an end bulb of a sciatic nerve and a cross-section of fibers of the lumbo-sacral cord (12). Another one of Mallory’s hematoxylin variants that gained traction in the 20th century was phosphotungstic acid hematoxylin (PTAH) and became more popular than its molybdic counterpart. One of PTAH’s first uses was by Masson in 1932, to selectively to stain the fibrils of Schwann cells and neurinomas blue (but not the ones from fibroblasts) (13). Perhaps the most prominent of the hematoxylin staining methods is the combination of hematoxylin and eosin (termed as H&E stain in current scientific literature) in which an acidic dye, eosin, and a basic dye, hematoxylin, are used together. The H&E was introduced around 1938 by Bailey and co-workers to stain the transverse and longitudinal sections of peripheral nerves, various different nerves in the body, and a variety of tumors within the body: meningeal tumor and an acoustic tumor (14). In 1929, Masson’s Trichrome Stain (Weigert’s Hematoxylin) was first used, and was further used in 1938 (15). Although the process of creating Trichrome stain and fixatives were well described but type of cells stained are lacking in these descriptions. The Trichrome stain with haemalum enable nuclei to be stained blue-violet, and cytoplasm are stained red while elastin is pink and collagen is golden yellow (15). The Trichrome stain with iron hematoxylin, process involves two principles (I) Mallory’s method of staining the entire sample with acid fuchsin and then differentiating them with PMAH, which helps discolor the collagen and bind the stain to the cytoplasm, and, (II) staining the collagen and cytoplasm red and yellow respectively, through the use of acid fuchsin in picric solution (the latter was originally developed by Van Gieson) (15). Masson’s Trichome was used to stain hypertrophied “Remak” bands, cytoplasmic nucleated cords, and axis cylinders and tumors (similarly to H&E). Despite developing over the course of the early and mid-1900s, most of these hematoxylin-containing stains are still in use today, either as nerve stains or simply as stains for other body parts. Böhmer’s hematoxylin, the first hematoxylin staining method developed, has not been used since the late 1800s but PTAH, PMAH, H&E, and Masson’s Trichrome stain all have found their usage to stain nerve tissues (16). PMAH and PTAH have been used to stain non-neuronal rather than neuronal tissues. The classic H&E stain is still in use to stain neuronal tissues (17). Additional strains, which were introduced over a century ago are para-phenylenediamine stain (PPD) and Nissl staining.
The PPD was used in 1919 by Menten to stain the medullary sheath of CNS neurons, where the PPD was combined with alpha-naphthol (18). Two improved modifications involved increasing the concentration of both alpha-naphthol and para-phenylenediamine and another was dissolution in salt solution or alcohol instead of water but they were pertinent to other tissues rather than neuronal tissue (18). PPD was used by itself in 1968 to stain cells in the CNS in preparation for electron microscopy (19). PPD continues to be used as a nerve stain, for example, to stain sections of human cranial nerves in order to rapidly count axons in the CNS (20).
Like PPD, the Nissl staining method has also been in use for over a century. This staining method involves the use of Cresyl violet acetate. In 1898, PPD was used in conjunction with H&E to stain the leptomeninges, external pia, and sections of the cerebral cortex (21). It was also used to stain extra-nuclear protoplasm and small sections from the occipital and frontal lobes (22). In 1934, the Nissl staining was used to highlight ganglion cells in the CNS (23). Nissl continues to be used today as a staining reagent for neuronal tissue (24).
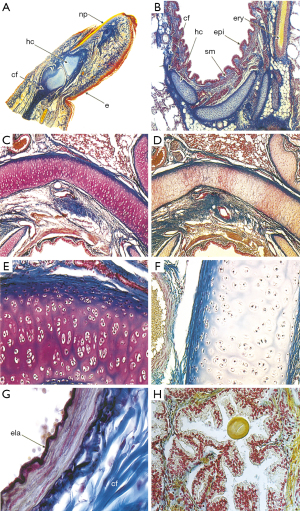
Carmine is a staining reagent that covers a broad spectrum of tissues. Beginning in the early 1910s, carmine staining techniques were used to stain the nuclei of small cells within degenerating nerve tissue. In early research, carmine was used to determine the location of Schwann cells (Figure 3). Microscopy at that time was not advanced. However, the carmine labeling of Schwann cell was helpful to describe corresponding regions as contributory to action potential as observed by Laidlaw in 1930. The degeneration and regeneration of medullated nerve fibers were also explored using this reagent (12). To track the induced nerve degeneration in medullated nerve fibers of domestic fowl, carmine and hematoxylin were used. Both carmine and hematoxylin stained the nuclei of the myelin sheath of Schwann cells within the PNS. This combination of staining reagents can detect extremely small cellular nuclei. A combination with Müller fixation method greatly reduced the size of the cells (12). The resting nerve fibers of Sepioteuthis sepioidea were investigated using such staining methods in a novel manner (25). To increase the visibility of potential difference levels of nerve fibers, carmine was inserted into the nerve tissue by the process of electrophoresis (26). A micropipette with carmine was used injected within the nerve in order to record the potential difference levels. The carmine injection appeared as a small spot of dye located within the Schwann cells, close to the nucleus and within the axon. When examining the Schwann cell and axon’s electrical potential difference levels, the carmine spot acted as a biomarker. The carmine was used to track the movement and conformation changes associated with electrical potential difference levels. Carmine was mixed with gelatin to form a gel. When inserted into the blood vessels, this preparation increases visualization of the cardiovascular system and its interchange with the nervous system. This gel allowed detection of alkaline phosphatases, the visibility of the blood vessels and their interaction with the surface of the nervous tissue (26). Although general carmine staining transitioned to use in different areas of medicine, new carmine staining techniques have evolved since the creation of this staining method which are heavily used to stain elements of the nervous system: alum carmine, mucicarmine and indigo carmine. The alum carmine (a combination of carmine and aluminum potassium sulfate) is most documented and used to counterstain the myelin sheath to detect nerve regeneration. In 1942, one of the first papers using alum carmine was published that enabled staining of the myelin sheath (27). The varying types of carmine are renamed based on the type of cell it stains or the other substance combined with carmine (Figure 3). The new methods provide a more targeted approach to specific cells, such as, mucicarmine’s ability to stain only mucin cells and indigo carmine’s use in spinal X-rays (28). As mentioned, the use of carmine staining techniques has shifted away from the nervous system to other tissues, mostly to detect variation in cell types. The human fetus finger is stained using carmine (Figure 4A) (29). The Figure 4B-4H highlight carmine staining within various cross sections, thus illustrating the diverse tissue types in which carmine staining can build contrast. Thus, carmine staining is still useful in neuronal staining. We note that the Azocarmine is not related to carmine and should not be grouped with this set of neuronal dyes.
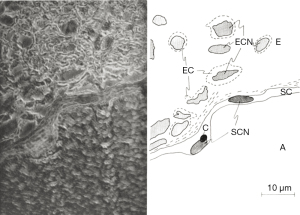
The general use of silver staining has been recorded in the scientific community since the early 1900s and continues to serve as a key staining reagent in the field of neuronal regeneration and degeneration. Some of the first recorded uses of the silver staining reagent was to enhance visualization of endoneurium fibers that surround each nerve cell (30). Endoneurial fibers are formed in layers that consist of connective tissue that surround the myelin sheath within the PNS. Although these fibers form layers of connective tissue, they also cluster into web-like formations that allow for the myelination of the nerve fibers. In 1930, Laidlaw used silver staining to target the endoneurial fibers of a cerebrospinal nerve and the sciatic nerve of a cat (30). The endoneurial fibers are stained dark black creating contrast from the invisible axis cylinders, Schwann cells and myelin sheaths (Figure 5). Before the development of silver staining other methods could target endoneurial fibers. However, they lacked ability to distinguish web sheathing. Silver staining techniques revolutionized observations of endoneurial webs can be observed. Because of hematoxylin and carmine staining’s inability to create clear resolution of tissue, Camillo Golgi created a new method of staining in hope to fully visualize the entire neuron (31). Golgi used silver nitrate in conjunction with previous techniques to enhance the clarity of neurons within tissue samples. Silver (and gold) has the ability to precipitate metal salts onto specific structures within samples, which greatly differs from past methods involving dyes instead of metals, such as those used in Nissl and hematoxylin staining (31). Silver staining is conducted by impregnating neural tissue with silver nitrate that crystallize within the membrane of cells. This method targets the soma and dendrites of a nerve cell. The impregnated cells are black, whereas the remaining tissue sample turns a light-yellow. Because of the black color of the stained neuron this method is commonly referred to as the “dark reaction”. Although this method allowed Golgi to illustrate the entire neuron beginning at the dendrite and ending with the cell’s soma, his method had flaws: extremely dark tissue samples, ability to stain blood vessels and its intensity of metallic properties, and specificity for young and embryonic human brain (31). Due to these inefficiencies, several attempts were made to better stain adult human brains (32). Santiago Ramon y Cajal, proposed two modifications of the Golgi method: (I) reduced silver impregnation and (II) gold chloride-sublimate impregnation. Both variations of the Golgi method for silver impregnation involved the use of metals instead of dyes for visualization. Cajal’s reduced silver impregnation method was suitable for studying neurofibrils and gold chloride-sublimate impregnation was for studying astrocytes (cell body’s stained gold, apical dendrite and basal dendrites of pyramidal cells stained black) (32). The Golgi method used ~200 µm thick tissues whereas Cajal’s modification reduced the tissue thickness to 100 µm producing clearer results.

The modifications by Golgi and Cajal revolutionized the silver impregnation method around that historic period (30). The large metallic interferences present in the Golgi method were greatly reduced by this process. Due to the specificity and contrast sensitivity this method is continued to be used even today, one of the latest being for measure of dendritic spine density in neural striations and the frontal cortical in mice (33).
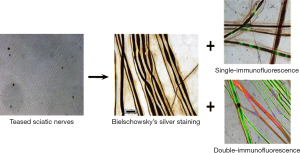
In the 1990s, this method of silver staining grew in popularity to better visualize cells. Due to the advancement of technique and the growth of the scientific community over time, prior procedures evolved into more efficient methods. Original silver impregnation method involved extremely complex steps that restricted their use (34). A simple and rapid method for the impregnation of neuronal bodies (34) rendered it applicable to methacrylate embedded sections though this modification also stained some glial bodies in the nervous system, though due to their density, the staining of glial cells did not affect targeting the neurons (34). Silver staining in conjunction with immunofluorescence is promising for enhancing the visualization within nervous tissue. The use of silver staining with either single- or double-immunofluorescence are now being attempted (Figure 6) (35) Because of the original silver staining’s inability to visualize specific features of the neuron, its use in conjunction with immunofluorescence has revived this now almost obsolete staining method. These utilizations include for assessment of neuronal degeneration and regeneration (36).
Aniline blue reagent is composed of methyl blue, water blue, or a mixture of both, that had one of its first uses in 1932 was by Masson to stain longitudinal fibrils in the sheath that encloses the cylinder. It was used to stain the thin pellicle that occupies the meshes in a reticulin web found around the cylinder, amorphous substance that separates the cylinders in a Schwannoma as well as collagen fibrils that surround tumor cells. This was done to challenge the idea that the endoneurium comes from mesodermal cells or Schwann cells. It was conjectured that the basement membranes are formed by the epithelium instead of the Schwann cells (13). Aniline blue was also used to stain a homogeneous glossy material that appeared between an unbroken row of cells in the stratum germinativum. These approaches were to determine whether peripheral nervous tumors originate from connective tissue or from Schwann cells (14). Aniline blue is used by itself as a tissue stain, but it is also incorporated as a component of other staining reagents. Recently, aniline blue has been used to stain components of the nervous system (37).
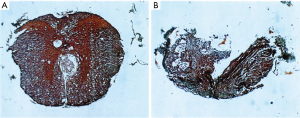
Mallory’s stain is an example of a stain that is composed of aniline blue as well as other chemicals (acid fuchsin and orange G). The Stroebe’s staining method involves the use of Muller’s fluid as a fixative in contrast a method developed by Mallory utilize formalin and Bouin’s fluid prior to staining with aniline blue (38). Mallory’s stain is also notable because his stain involved the use of PTA and PMA. The researchers that used aniline blue in the past period had not included these reagents. This is the reason that Mallory’s contribution to the aniline blue staining method continues to bear his name even today. One of the first uses of Mallory’s stain in nerve tissue was in 1942 for the purpose of determining the relationship between the time taken between sutures and the effectiveness of nerve regeneration by columns of Schwann cells (27). Mallory’s stain was useful in visualizing degenerated cells in mitosis. This stain also allowed for the differentiation of many organelles within Schwann cells, such as cytoplasm (s.c.) and nucleus (s.n.); as well as the axons (ax.) and collagen (c.) (Figure 7). Mallory’s stain has been used recently to stain anterior optic nerves in order to visualize the damage due to elevated intraocular pressure (39). Recently this stain has also allowed for the reconstruction of 3D images revealing different pathological features in a spinal cord compression injury (Figure 8) (40).

Challenges and difficulties in the use of staining reagents for neuronal tissue
Despite the great utility of histological stains, they have limitations. The staining reagents such as mucicarmine and PPD, had difficulties in preparation during initial stages of development but were effective once the preparation procedures matured. The mucicarmine was developed using two methods, Meyer’s and Southgate’s, and in both methods the stain could potentially be damaged during the creation due to the heat applied during its preparation (28). Similarly, PPD was difficult to use due to it quick decomposition if not made immediately before utilized. Additionally, when PPD was combined with alpha naphthol, researchers struggled to develop the correct ratio of the mixture that would be most effective (18). Like PPD, picric acid also requires a counterstain in order to achieve visualization, eventually hematoxylin was found to be suitable counter stain (9). Other stains, such as hematoxylin, PMAH, carmine and Cajal’s silver, caused shrinkage of the tissue when hardened in absolute alcohol and thus made it very difficult to discern which sections were being stained and which were not (12). Shrinkage is not the only type of damage. The H&E when used to stain the perineurium results in loosening of the inner surface of the perineurium and tissue disintegration (14). Finally, another drawback of histological stains is inconsistency in the degree to which they stain the same type of tissue. For example, Golgi’s method was useful for embryonic and young brains but not for those of adults or human brains (32) while, PTAH only stained a few of the fibrils in neurinomas blue and not all of them (13). This was also the case with silver chromate which only stained the cell body and ascending filament or the descending filament of bipolar cells, but not the cell as a whole (31). Although various difficulties were encountered throughout history when using and developing histological stains, however, they conferred the ability to visualize tissue details that would not be possible otherwise.
Overview
Development of comprehensive small molecule staining reagents/methods
We prepared a comprehensive small molecule staining reagents list (https://cdn.amegroups.cn/static/public/aes-21-31-1.xlsx). We used search keywords such as: “regeneration”, “degeneration”, and “nerve regeneration” in English, German (nervenregerneation), and French (degeneration) on Google Scholar, PubMed, and Archive. We filtered the results by narrowing the date of publication to 1700–1950, in order to find the first use of each staining reagent. We focused on this timeframe because the first use of a small molecule staining reagent was in the 1700s whereas, immunochemistry based staining reagents were developed in the 1950 onwards. Since the focus of this review is on small molecule staining reagents, we decided to narrow our search to 1700–1950. Additionally, we focused on the languages English, German, and French. The neuroscience researchers who used staining in the period 1700–1950 published their work in these languages. We next searched each publication for the keyword in English (“stain”), German (“farbung”), and French (“reactif”) depending on the language of the article. Many reagents were used in several published reports. After three rounds of exhaustive search, we next focused on the reagents found and searched for their chemical and/or biochemical properties and the CNS, PNS as well as specificity for staining organelles (https://cdn.amegroups.cn/static/public/aes-21-31-2.xlsx).
Biochemistry of small molecule staining reagents
Based on how the reagents act on a tissue to stain the organelles can be grouped into different categories. The most common grouping was whether the small molecule staining reagent binds to acidic or basic portions of the organelle (Figure 9). A reagent that was included in this grouping was ponceau-fuchsin because it binds to positively charged (basic) functional groups of proteins as well as non-polar regions of the protein. On the other hand, toluidine blue preferentially stains acidic tissue components, so we also grouped it into this category. Other groupings include the reagents binding through an oxidation-reduction reaction and hydrogen bonding. Reagents that bind via an oxidation-reduction reaction include osmic acid, hematoxylin and eosin (H&E staining), and periodic-acid Schiff. Osmic acid forms a black reduction compound with fats by the addition to an alkene bond, while both H&E staining and periodic-acid Schiff react via oxidation reactions. Carmine and alum carmine both bind to glycogen via hydrogen bonding. There were also reagents that could not be grouped into a category because there was not an overlap in the reactions that take place in order for them to stain the tissue. Examples of such reagents are: aniline blue, Bodian’s protargol stain, phosphotungstic hematoxylin, Golgi method, Böhmer’s hematoxylin, picric acid-hematoxylin method, ranson pyridine silver staining, herxheimer’s scarlet red (Sudan IV), orange G, Schiff's plasmal reagent, Bielschowsky, Verhoeff’s elastic stain, and paraldehyde fuchsin.

Molecules, tissues, and organelles stained within the nervous system
The small molecule staining reagents may be used to stain molecules, tissues, and organelles within cells that comprise the neuronal systems. Acid mucopolysaccharides and radicals are examples of molecules that may be stained. Tissues within the nervous system that can be stained with these reagents include normal and degenerating myelin, Schwann cells, glial cells and neurons. The organelles that may be stained with these small molecule staining reagents include but are not limited to the: nucleus, nucleoli, cytoplasm. In addition, structures like axis cylinders could also be stained (https://cdn.amegroups.cn/static/public/aes-21-31-3.xlsx).
Future use of staining reagents
In recent years, there has been a shift from the use of small molecule staining reagents to more specific means of visualization (immunohistochemistry or immunocytochemistry) using antibodies either directly conjugated to fluorophores or detected using fluorophore conjugated secondary antibodies to primary. The small molecule staining reagents provided fundamental groundwork to the discoveries of organelles and processes within the nervous system, their relevance in emerging science has since then declined. The limitations posed by small molecule reagents staining is lack of specificity, a limited number of cellular components/limited visualization. However, integrating immunohistochemistry with staining reagents enable simultaneous, targeted imaging to an antigen and mapping tissue composition (41). Combining antibodies to presynaptic marker SV2A along with α-bungarotoxin (α-BTX) have enabled the post-synaptic acetylcholine receptors (AChRs) in muscles (42). Combinations of small molecule fluorophores expands our analytical reach to in situ hybridization for example, complementary nucleic acid hybridization probes to perform single-molecule fluorescence in situ hybridization (smFISH) (43). Most recently the small molecule staining reagents have been used in lipid and metabolite profiling, for example metabolic changes in hippocampal samples of traumatic brain injuries (44). Using isobaric exchanges (for example deuterium in deuterated water) in kinetics histochemistry (45) the pre-existing versus newly formed metabolites (or lipids) may be identified. Combining with staining reagents may further expand these analytical abilities. The combined use of small molecule staining, fluorescent labeling and tissue clearing may render the visualization of spatial relationships between neurons pathologic structures such as amyloid plaques in Alzheimer’s disease (46).
In summary, neuronal staining methods have shifted from the use of small molecule staining reagents to primarily fluorophore conjugated antibody mediated immunohisto- or immunocyto-chemistry as well as the new technique of imaging mass spectrometry. The use of small molecule staining reagents is not over yet. The small molecule staining reagents still retains the utility to be used in conjunction with other reagents and methods such as kinetic histochemistry to derive greater amount of information for vision science and ophthalmology.
Acknowledgments
Funding: This work has been partly supported by an unrestricted grant from Research to Prevent Blindness and NIH grants EY14801, EY031292. All illustrations in the paper have been drawn using Biorender.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-21-31/coif). SKB serves as an unpaid editorial board member of Annals of Eye Science from August 2020 to July 2022. The authors report that their research is mostly online using public databases. However, infrastructure such as basic software including zoom meetings were supported by the University of Miami. Personal and software has support from NIH grant EY14801, EY031292 (SKB) and RPB unrestricted grants to University of Miami for research.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020;25:1340. [Crossref] [PubMed]
- Baloyannis SJ. Staining neurons with Golgi techniques in degenerative diseases of the brain. Neural Regen Res 2015;10:693-5. [Crossref] [PubMed]
- Sulaiman RS, Merrigan S, Quigley J, et al. A novel small molecule ameliorates ocular neovascularisation and synergises with anti-VEGF therapy. Sci Rep 2016;6:25509. [Crossref] [PubMed]
- Calkins DJ, Pekny M, Cooper ML, et al. The challenge of regenerative therapies for the optic nerve in glaucoma. Exp Eye Res 2017;157:28-33. [Crossref] [PubMed]
- Goldberg JL, Guido W. Agi Workshop Participants. Report on the National Eye Institute Audacious Goals Initiative: Regenerating the Optic Nerve. Invest Ophthalmol Vis Sci 2016;57:1271-5. [Crossref] [PubMed]
- Benowitz LI, He Z, Goldberg JL. Reaching the brain: Advances in optic nerve regeneration. Exp Neurol 2017;287:365-73. [Crossref] [PubMed]
- Arnemann J. Ueber die Reproduktion der Nerven. Göttingen, J.C. Dieterich, 1786.
- Versuche über die Regeneration an lebenden Thieren: Über die Regeneration der Nerven : Mit IV Kupfertafeln. Göttingen, J.C. Dieterich, 1787.
- Howell WH, Huber GC. A Physiological, Histological and Clinical Study of the Degeneration and Regeneration in Peripheral Nerve Fibres after Severance of their connections with the Nerve Centres. J Physiol 1892;13:335-406.11.
- Böhmer F. Zur pathologischen Anatomie der Meningitis cerebromedullaries epidemica. 1865 Intelligenz-Blatt 539-50.
- Eltoum I, Fredenburgh J, Myers RB, et al. Introduction to the Theory and Practice of Fixation of Tissues. J Histotechnol 2001;24:173-90. [Crossref]
- Clark E. Regeneration of medullated nerves in the absence of embryonic nerve fibers, following experimental non-traumatic degeneration. J Comp Neurol 1914;24:61-111. [Crossref]
- Masson P. Experimental and Spontaneous Schwannomas (Peripheral Gliomas): II. Spontaneous Schwannomas. Am J Pathol 1932;8:389-416.11.
- Bailey P, Herrmann JD. The rôle of the cells of Schwann in the formation of tumors of the peripheral nerves. Am J Pathol 1938;14:1-38.19.
- Masson P. Some histological methods. Trichrome stainings and their preliminary technique. Available online: https://www.scienceopen.com/document?vid=c238231e-0cf4-455e-9279-b1416209106f
- Türedi S, Yuluğ E, Alver A, et al. A morphological and biochemical evaluation of the effects of quercetin on experimental sciatic nerve damage in rats. Exp Ther Med 2018;15:3215-24. [PubMed]
- Ariel de Lima D, Helito CP, Lacerda de Lima L, et al. Study of the Nerve Endings and Mechanoreceptors of the Anterolateral Ligament of the Knee. Arthroscopy 2019;35:2918-27. [Crossref] [PubMed]
- Menten ML. A Study of the Oxidase Reaction with alpha-Naphthol and Paraphenylenediamine. J Med Res 1919;40:433-458.3.
- Berkowitz LR, Fiorello O, Kruger L, et al. Selective staining of nervous tissue for light microscopy following preparation for electron microscopy. J Histochem Cytochem 1968;16:808-14. [Crossref] [PubMed]
- Engelmann S, Ruewe M, Geis S, et al. Rapid and Precise Semi-Automatic Axon Quantification in Human Peripheral Nerves. Sci Rep 2020;10:1935. [Crossref] [PubMed]
- Stewart P. General Paralysis of the Insane During Adolescence, with Notes of Three Cases. Brain 1898;21:39-57. [Crossref]
- Wright HK. The Cerebral Cortical Cell Under the Influence of Poisonous Doses of Potassii Bromidum. Brain 1898;21:186-223. [Crossref]
- Grayzel DM. Changes in the Central Nervous System Resulting from Convulsions due to Hyperinsulinism. Arch Intern Med 1934;54:694-701. [Crossref]
- Fang YY, Zeng P, Qu N, et al. Evidence of altered depression and dementia-related proteins in the brains of young rats after ovariectomy. J Neurochem 2018;146:703-21. [Crossref] [PubMed]
- Villegas R, Villegas L, Gimenez M, et al. Schwann cell and axon electrical potential differences. Squid nerve structure and excitable membrane location. J Gen Physiol 1963;46:1047-64. [Crossref] [PubMed]
- Nóblega HG, Missaglia V, Stenert C, et al. Vascular supply of the central nervous system of the land snail Megalobulimus oblongus (Gastropoda, Pulmonata). Braz J Med Biol Res 2003;36:1247-53. [Crossref] [PubMed]
- Holmes W, Young JZ. Nerve regeneration after immediate and delayed suture. J Anat 1942;77:63-96.10.
- Dapson RW. The history, chemistry and modes of action of carmine and related dyes. Biotech Histochem 2007;82:173-87. [Crossref] [PubMed]
- Arend A, Kolts I. Carmine-picroindigocarmine: an alternative multiple staining method. Ann Anat 2002;184:149-52. [Crossref] [PubMed]
- Laidlaw GF. Silver Staining of the Endoneurial Fibers of the Cerebrospinal Nerves. Am J Pathol 1930;6:435-444.3.
- Levine C, Marcillo A. Origin and endpoint of the olfactory nerve fibers: as described by Santiago Ramón y Cajal. Anat Rec (Hoboken) 2008;291:741-50. [Crossref] [PubMed]
- Andriezen WL. A Modified Golgi's Method for the Study of the Human Brain. Br Med J 1894;1:909. [Crossref] [PubMed]
- Nagarajan N, Jones BW, West PJ, et al. Corticostriatal circuit defects in Hoxb8 mutant mice. Mol Psychiatry 2018;23:1868-77. [Crossref] [PubMed]
- Tolivia D, Tolivia J. A new rapid silver impregnation for neuronal bodies on methacrylate sections. J Neurosci Methods 1991;36:139-43. [Crossref] [PubMed]
- Segura-Anaya E, Flores-Miranda R, Martínez-Gómez A, et al. A novel histochemical method of simultaneous detection by a single- or double-immunofluorescence and Bielschowsky's silver staining in teased rat sciatic nerves. J Neurosci Methods 2018;304:46-51. [Crossref] [PubMed]
- Lavenir I, Passarella D, Masuda-Suzukake M, et al. Silver staining (Campbell-Switzer) of neuronal α-synuclein assemblies induced by multiple system atrophy and Parkinson's disease brain extracts in transgenic mice. Acta Neuropathol Commun 2019;7:148. [Crossref] [PubMed]
- Hebbard P, Ivanusic J, Sha S. Ultrasound-guided supra-inguinal fascia iliaca block: a cadaveric evaluation of a novel approach. Anaesthesia 2011;66:300-5. [Crossref] [PubMed]
- Huber GC. A study of the operative treatment for loss of nerve substance in peripheral nerves. J Morphol 1895;11:629-740. [Crossref]
- Knox DL, Eagle RC Jr, Green WR. Optic nerve hydropic axonal degeneration and blocked retrograde axoplasmic transport: histopathologic features in human high-pressure secondary glaucoma. Arch Ophthalmol 2007;125:347-53. [Crossref] [PubMed]
- Duerstock BS. Double labeling serial sections to enhance three-dimensional imaging of injured spinal cord. J Neurosci Methods 2004;134:101-7. [Crossref] [PubMed]
- Bishop DP, Cole N, Zhang T, et al. A guide to integrating immunohistochemistry and chemical imaging. Chem Soc Rev 2018;47:3770-87. [Crossref] [PubMed]
- Jonsson S, Wiberg R, McGrath AM, et al. Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery. PLoS One 2013;8:e56484. [Crossref] [PubMed]
- Gonzales BJ, Mukherjee D, Ashwal-Fluss R, et al. Subregion-specific rules govern the distribution of neuronal immediate-early gene induction. Proc Natl Acad Sci U S A 2020;117:23304-10. [Crossref] [PubMed]
- Li T, Hu E, Li P, et al. Metabolomics Deciphers Potential Targets of Xuefu Zhuyu Decoction Against Traumatic Brain Injury in Rat. Front Pharmacol 2020;11:559618. [Crossref] [PubMed]
- Louie KB, Bowen BP, McAlhany S, et al. Mass spectrometry imaging for in situ kinetic histochemistry. Sci Rep 2013;3:1656. [Crossref] [PubMed]
- Vints K, Vandael D, Baatsen P, et al. Modernization of Golgi staining techniques for high-resolution, 3-dimensional imaging of individual neurons. Sci Rep 2019;9:130. [Crossref] [PubMed]
Cite this article as: Rosario A, Howell A, Bhattacharya SK. A revisit to staining reagents for neuronal tissues. Ann Eye Sci 2022;7:6.


