
Inflammatory pathways in pathological neovascularization in retina and choroid: a narrative review on the inflammatory drug target molecules in retinal and choroidal neovascularization
Introduction
The retina works with a high metabolic demand, and vascular maintenance is vital to sustaining normal metabolic function, visual processing and retina homeostasis (1). Improper vascular maintenance and/or blood supply affect normal visual function and cause disease conditions. Dysregulated angiogenesis is one pathological aspect of vision-impairing retinal diseases, such as diabetic retinopathy (DR) and age-related macular degeneration (AMD). In these diseases, neovascularization (NV) accelerates structural and functional damage in the retina (asterisks in Figure 1). At the early and intermediate stages of DR and AMD, prior to NV, there are activated endothelial cells (ECs) and immune cells, local and systemic inflammatory responses, and retina degeneration (2).
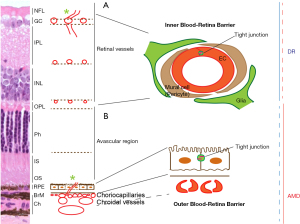
DR and AMD are the leading causes of the blindness among working adults, and elderly population, respectively. The prevalence of diabetes mellitus (DM) was 442 million in 2014 and continues to increase throughout the world (3). More than one-third of DM patients develop DR, one of the most common complications (4,5). DR patients may appear asymptomatic at early stages, but the ongoing progression of leukocyte adherence, capillary dropout, and mural cell activation and loss can advance to a later stage known as proliferative DR (PDR). PDR is characterized by retinal NV at the vitreoretinal interface and/or the inner retina (Figure 1A). In contrast, AMD, a multifactorial degenerative disorder of the central retina, advances to geographic atrophy and/or choroidal NV (Figure 1B). DR and AMD are characterized by the loss of inner and outer blood retina barrier (BRB) integrity, respectively. Inner BRB is maintained by tight junctions of endothelia, mural and glial cells (Figure 1A), whereas outer BRB is maintained by tight junctions of retinal pigment epithelium (RPE) and Bruch’s membrane (BrM) (Figure 1B).
In this review article, we aim to describe and discuss the pathological aspects of cells associated in inner and outer BRB and retinal and choroidal NV, as well as the role of inflammatory molecules. Finally, we will discuss current advances in the therapeutic targets for retinal and choroidal angiogenic conditions. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aes-21-4)
Cells in retinal and choroidal NV
The final effector cells in angiogenesis are ECs, but the main pathological cells that initiate the process are mural and RPE cells in retinal and choroidal NV, respectively. Mural and RPE cells are essential in maintaining the inner and outer BRB. Further, there are many cell types associated with the pathological processes of retinal and choroidal NV. We categorize them for the convenience of description: EC and mural cell, RPE, glial cells, and microglia and macrophages.
Cells in retinal and choroidal NV: vascular EC and mural cell
Retinal vasculature consisting of ECs, basement membrane and mural cells, is the essential component for inner BRB and protects the inner retina (Figure 1A). Retinal blood vessels contain an abundance of mural cells (1:1 ratio with EC), which is quite uncommon in other body regions. For an example, the varying ratio of mural cell to EC is 1:100 in skeletal muscle (6,7). Mural cells support EC survival and vascular stability, inhibiting angiogenesis in normal retina (8). During DR progression, the loss of mural cells is an early feature in retinal capillaries (9), causing capillary dilation, microaneurysms, leakage and edema, as well as EC death, leading to vascular closure and eventual retinal NV (8,10,11). There are some notable differences in fenestration between retinal and choroidal vasculatures. The choriocapillaris (innermost layer of choroid) is highly permeable and consists of only ECs, with very little basement membrane and mural cells (12,13) (Figure 1B), whereas RPE contains tight junctions which protect the outer neural retina.
Cells in retinal and choroidal NV: RPE
RPE is a polarized monolayer, with apical microvilli processes adjacent to the photoreceptors and a folded basal aspect on the BrM (Figure 1B). RPE tight junctions, located apical-laterally to the adjacent cells, provide an essential property of the outer BRB. RPE controls the passage of ions, water, and metabolites between the retina and choriocapillaris, and takes up nutrients, such as glucose and fatty acids, from the choroid to nourish photoreceptors, and removes metabolic wastes from the photoreceptors to the choriocapillaris. RPE, also participates in the visual cycles, providing 11-cis retinal to photoreceptors and taking all-trans retinal from photoreceptors (14,15). RPE dysfunction, therefore, disturbs the healthy outer retinal environment. Aged RPE increases lipofuscin and basal deposits, forming drusen. In AMD, RPE is a major degenerated cell type and secretes vascular endothelial growth factor (VEGF), an angiogenic molecule, and the increased VEGF induces choroid NV (14) (Figure 2). RPE disturbance, along with reduced phagocytic and electrophysiological function, is observed in DR as well (16-18) (Figure 2).
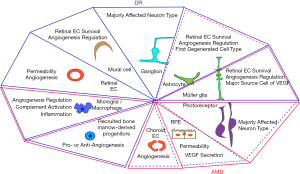
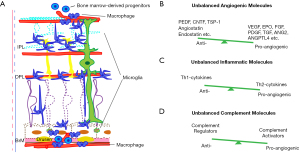
RPE secretes a variety of growth factors to support photoreceptors and ECs and regulate angiogenesis: fibroblast growth factor (FGF), transforming growth factor β (TGFβ), ciliary neurotrophic factor (CNTF), pigment-epithelium-derived factor (PEDF) and VEGF, etc. FGF2 (19) and TGFβ (20) are upregulated in RPE/choroid tissue of choroidal NV, and the inhibition of FGF2 (21,22) and TGFβ (20,23) prevents the NV formation in animals. However, there are controversial observations that the absence of TGFβ signaling in microglia (24) or EC (25) exacerbates choroidal NV. Therefore, TGFβ function is either pro- or anti-angiogenic, depending on the intracellular signaling pathways and the cell types (26). CNTF (27) and PEDF (28), secreted from the apical surface of RPE, support photoreceptor survival and suppress NV in retina, while VEGF is mainly secreted from the basolateral surface of RPE to support choroid (28,29). VEGF expression decreases with age but continues to persist (30-32). Furthermore, RPE secretes cytokines and chemokines, such as monocyte chemoattractant protein 1 (MCP1), in a polarized manner (33,34), recruiting leukocytes and amplifying inflammation (Figure 3A). The balance and polarized secretion of anti- and pro-angiogenic and inflammatory molecules maintains a healthy outer retinal environment (Figure 3B-3D).
Cells in retinal and choroidal NV: astrocytes and Müller glia
Astrocytes and Müller glia in neurovascular units of the retinal vascular plexus (Figure 1A) are altered and associated with pathogenesis of retinal and choroidal NV (Figure 2). Astrocytes located in nerve fibers and ganglion cell layers are initially damaged before Müller cell activation during DR progression (35,36) and astrocyte alteration is also reported in AMD patients (37,38) (Figure 2). Müller glia, located across the entire retina, support and modulate all neuron types in the retina and is the major cell type to secrete the pro-angiogenic molecule VEGF, in DR (15,39,40). Müller glia activation and alteration are also observed in AMD: Glial fibrillary acidic protein-positive Müller glia processes are extended to the outer limiting membrane (41,42), and are sprouted on the vitreal surface of the inner limiting membrane (38) (Figure 2).
Cells in retinal and choroidal NV: microglia and macrophages
Microglia, the retina resident immune cell type (Figures 2,3A), are normally found in two plexiform layers, but they are relocated to damaged areas (43) (Figure 3A). The activated microglia produce inflammatory cytokines and other molecules, such as metalloproteases and nitrous oxide, which potentiate chronic pathological inflammation (44). There is further direct evidence that microglia are involved in NV: the lack of microglia exhibits a reduced vascular density (45,46) and co-culture of microglia and aortic ring enhances the vascular branches from the ring (47). The NVs recruit cells from peripheral blood stream and bone marrow as well (48-50) (Figures 2,3A). The resident microglia and recruited monocytes and macrophages activate into two subgroups: M1 and M2 microglia and macrophages, and neutrophils activate into, typically, N1 and N2 (51-53). M1 microglia and macrophages, and N1 neutrophils secrete Th1-derived cytokines, such as interferon (IFN)-γ, whereas M2 macrophages and N2 neutrophils secrete Th2-derived cytokines, such as IL-4. IL-4 is either pro-angiogenic (54) or anti-angiogenic (55,56) (Figure 3C). M2 macrophages and N2 neutrophils additionally secrete pro-angiogenic molecules, such as VEGF and matrix metalloprotinase 9 (51,57-59) (Figure 3C).
Besides local unbalanced inflammation, peripheral inflammation is also associated with DM/DR and AMD. The deletion of peroxisome proliferator activated receptor-γ (PPAR-γ), a nuclear receptor transcription factor that mediates M2 macrophage phenotype (60), contributes to obesity and insulin resistance (61,62). On the other hand, choroidal NV patients have higher counts of macrophages and neutrophils, and elevated levels of inflammation markers, such as C-reactive protein, and platelets in blood (63,64). VEGF+ M2 activated macrophages are peripherally observed at the initial choroidal NV stage (65) as well, and CCR2 monocyte depletion suppresses choroidal NV formation (66). Transgenic ccl2, ccr2, cx3cr1 and ccl2/cx3cr1 deficient mice are associated with aberrant monocyte trafficking and exhibit choroidal NV features with other AMD phenotypes (67-70). Finally, peripheral monocyte depletion induced by clodronate (48) and neutrophil depletion (CC chemokine receptor 2 knockout mice) (66) result in a reduced size of laser-induced choroidal NV, indicating the direct contribution of peripherally circulating immune cells in the NV process.
Inflammatory pathways in retinal and choroidal NV
It is well known that inflammatory pathways are highly activated in retinal and choroidal NV, owing to dysregulation of complement activities in DR and AMD (71,72), as well as increased environmental risk factors such as obesity, hypertension, smoking and high-fat diet (73-76). Genetic studies have indicated that a dysregulated complement system is linked with DR (77,78) and AMD (72,79). The inflammatory features of local resident and recruited cells from peripheral blood stream and bone marrow contribute to the NV environment of DR and AMD. We review the current advances in inflammatory molecular pathways of the complement system, stromal derived factor-1 (SDF-1)/chemokine CXC receptor-4 (CXCR4), inflammasome nucleotide-binding oligomerization domain (Nod)-like receptor containing domain 3 (NLRP3), interleukin 18 (IL-18), programed cell death ligand-1 (PD-L1), insulin-like growth factor (IGF) and sphigosin-1-phosphate receptor (S1PR), to gain insights into potential candidates of therapeutic targets to treat retinal and choroidal NV.
Inflammatory pathways in retinal and choroidal NV: complement system
The complement system, part of the innate immune system, participates in pathogen elimination, but also becomes involved in diverse biological processes including cell differentiation, synaptogenesis, tissue clearance, degeneration, regeneration, lipid metabolism, tumorigenesis and angiogenesis (80). Activation of the complement system is accomplished through three pathways: classical, lectin, and alternative (Figure 4A). Classical pathway is associated with humoral immune system, activated by antigen-antibody binding, and lectin pathway is associated with innate immune system, activated by glycoproteins and glycolipids, whereas the alternative pathway is particularly implicated in sterile/pathogen-free inflammation of chronic neurodegenerative diseases (80,81).
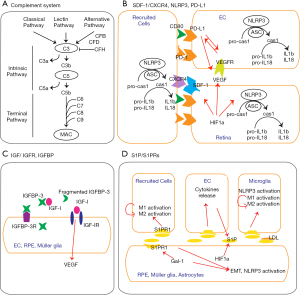
While the initiation of each pathway is distinct, all three eventually converge to produce C3 and C5 convertases (80,81). Complement factor B (CFB), complement factor D (CFD) and serine proteases are required for the generation of C3 convertase, while complement factor H (CFH) is the main inhibitor of C3 activation. The anaphylatoxins C3a and C5a are the chemo-attractants that guide monocytes, macrophages, neutrophils and the other immune cells. Complement activation is implicated in DR and AMD pathogenesis and pathobiology (71,81,82). In retinal and choroidal NV, there are high levels of complement activators, such as CFD, C3 and C5, and low level of regulators, such as CFH (Figures 3B and 4A). Resistance to streptozotocin (STZ)-induced diabetes was observed in C3 knockout mice (83), and the genetic association of C5 genes in type 2 diabetes and PDR was observed (78,84). Complement components are found in patient choroidal NV (71,72), and the deficiency of C3a and C5a receptors, as well as C3 and C5 knockouts, have shown reduced VEGF expression and resistance to laser-induced choroidal NV formation (85,86). In addition, the overexpression of C3 and C5 induces choroidal NV (86). However, mice lacking receptors for C3a and C5a show early onset of retinal degeneration, and were more susceptible to light-induced retinal dysfunction (87). These findings indicate that the complement system is a double-edged sword regarding tissue protection and degeneration, and should be tightly balanced: The plenty contributes to NV, but sparsity contributes to retina degeneration. Clinical trials have often attempted to target complement components, not only for geographic atrophy Dry AMD, but also for choroid NV Wet AMD (82,88). It is of note that while CFD inhibitor Lampalizumab (NCT02247479; NCT02247531, Phase 3, Genentech), C3 inhibitor Compstatin (NCT01157065, Phase 2, Apellis) and C5 inhibitor Eculizumab (NCT00935883, Phase 2, Alexion pharmaceuticals) failed in clinical trials to treat Dry AMD (89), c3 inhibitor APL-2 Pegcetacoplan (NCT03525600, NCT03525613, Phase 3, Apellis Pharmaceuticals) and c5 inhibitor (Zimura; NCT02686658; Phase 2/3, IVERIC bio) are currently in clinical trials and awaiting the results (90,91). IB1302 Bispecific antibody fusion protein targeting VEGF and complement cascade (NCT04820452, Innovent Biologics, Inc.) finished Phase 1 and just started Phase 2 for Wet AMD in April, 2021.
Inflammatory pathways in retinal and choroidal NV: stromal cell derived factor-1 (SDF-1)/chemokine CXC receptor-4 (CXCR4)
Chemokines and their cognate receptors are involved in the migration of peripheral cells to injury sites. SDF-1 (also known as chemokine ligand 12, CXCL12) and the receptor CXCR4 are considered an essential chemokine signaling pathway in NV (92) (Figure 4B). CXCR4 was initially cloned from leukocytes (93,94) and is cofactor for HIV-1 entry into T cells (95). Both SDF-1 and CXCR4 contain hypoxia response elements within the promoters and are induced by a major transcription factor, hypoxia-inducible factor-1 (HIF-1). SDF-1/CXCR4 axis is involved in recruitment and differentiation of hematopoietic progenitors to hypoxic sites. In either ischemic retinopathy or laser-induced choroidal NV, both SDF-1 and CXCR4 are up-regulated and co-localized in astrocytes, Müller glia, RPE, choroid (96,97), hematopoietic and bone marrow-derived CD45+ cells and microglia/macrophage, but not in ECs (97). CXCR4 blockade (97,98) reduces movement of bone marrow-derived CD45+ cells and F4/80+ macrophages into ischemic retina, and suppresses the formation of retinal and choroidal NV (97), but has no apparent regression effect for established choroidal NV (98).
Inflammatory pathways in retinal and choroidal NV: Nucleotide-binding oligomerization domain (Nod)-like receptor containing pyrin domain3 (NLRP3)
Chronic and sterile inflammation is a hallmark of chronic diseases, including DR and AMD. The inflammasomes, as one of the pattern recognition receptors in the innate immune system, regulate secretion of pro-inflammatory cytokines via caspase-1 activation, in response to endogenous damage and infectious signals. Inflammasome activation leads to conversion of procaspase-1 into active caspase-1, and the maturation of pro-interleukin (IL)-1beta and pro-IL-18 (Figure 4B). The NLRP3 (known as cryopyrin) is mostly characterized among other NLR inflammasomes and has been a growing area of interest, given its association with chronic degenerative and metabolic diseases (99,100). Upregulated activation of NLRP3 inflammasome was observed in macrophages, liver and kidney of diabetic patients (101-104), and in vitreous fluid and retina of DR patients (105,106), as well as animals (107). The attenuation of NLRP3 inflammasome activation and oxidative stress, as a result of treatment with IL-22, curcumin, cepharanthine or piperine or fenofibrate, has mitigated diabetic nephropathy (108-111), and recently, intravitreal injection of MCC950, a specific inhibitor of NLRP3 ameliorated retinal NV in an oxygen-induced ischemic retinopathy model (112). Similarly, AMD animal models of Dry and Wet forms have shown increased levels of NLRP3 inflammasome activation (113-116), and the clinical aspects of AMD, such as drusen and complement activation, suggest inflammasome’s association in pathophysiology conditions of AMD. However, it is still unclear how NLRP3 is directly associated with choroidal NV. Laser-induced choroidal NV in NLRP3 genetic deficiency exhibited exacerbation (114), but VEGF-Ahyper choroidal NV model in NLRP3 genetic deficiency exhibited decline (113). When it is considered that laser-induced acute model does not fully represent clinical Wet AMD pathophysiology and VEGF-Ahyper is relatively chronic, studies in other animal models such as knockout of very-low-density lipoprotein receptor (VLDLR) (117) may confirm NLRP3 association further. Since inflammation itself is neutral like weapon, NLRP3 inflammasome might take either protective or deleterious roles, depending on the stages and conditions of diseases, and the studies on the associated-downstream pathways and cell specific mechanisms will be important.
Inflammatory pathways in retinal and choroidal NV: interleukin (IL)-18
Proinflammatory IL-1beta and IL-18 are downstream cytokines of NLRP3 activation. Like NLRP3 upregulation in retinal and choroidal NV, as mentioned above, IL-1beta and IL-18 are also upregulated in aqueous and vitreous fluids of non-PDR and PDR (105,106,118), and are considered increased in choroidal NV patients as well based on cell and animal studies (114,115,119-121). The upregulation of IL-1beta seems to be associated with oxidative stress, BRB permeability and tissue deterioration in DR and AMD (113,114,121-125). However, there are controversial results of IL-18 function in retinal and choroidal NV, indicating the physiological complexity of IL-18 functions. Deficiency of IL-18 resulted in severe choroidal NV development and treatment of IL-18 attenuated choroidal NV formation via reducing VEGF in laser-induced choroidal NV model (114,119), and IL-18 deficiency in VEGF-Ahyper model increased choroidal NV compared to that in NLRP3 and IL-1beta deficiency in VEGF-Ahyper (113). Interestingly, NLRP3 and IL-1beta targeting resulted in inhibition of Dry AMD pathology but IL-18 inhibition deteriorated AMD pathology in CEP-adducted serum albumin-immunized model. However, any deficiency of either IL-1beta, NLRP3 or IL-18 did not inhibit choroidal NV formation in VEGF-A hyper model. It was later argued that IL-18 does not exhibit either pro- or anti-angiogenic effects on laser-induced choroidal NV formation (120,126). Nevertheless, anti-VEGF treatment increases ocular IL-18 levels, and the increased IL-18 level is correlated with good visual outcome in patients with macular edema. Similarly, upregulation of IL-18 by anti-VEGF treatment occurs in ischemic retinopathy animal model, suggesting anti-angiogenic activity of IL-18 (127). It is noteworthy that the expression level of pro-IL-18 and IL-18 is known to be permanent in RPE cells, and not increased by NLRP3 inflammasome activity (125). It is also well known that endothelium and epithelium cells are hardly activated by inflammation, thus the cytokine roles of resident and recruited immune cells will be important. Finally, the level of IL-18 concentration may have a protective role in retinal and choroidal NV, whereas higher amounts of IL-18 combined with other cytokines, may be detrimental.
Inflammatory pathways in retinal and choroidal NV: programed cell death ligand-1 (PD-L1)
Programmed cell death receptor (PD-1) and its ligand PD-L1, immune checkpoint proteins, are essential in ocular immune privilege. PD-L1 is found in ocular tissues, including ECs and RPE, and PD-L1 expression is increased under inflammatory conditions (128,129). PD-1 is expressed on lymphocytes and antigen presenting cells. PD-L1 blocking/downregulation using anti-PD-L1 antibody and siRNA treatment enhances pro-inflammatory cytokine production (128) and increases EC proliferation with upregulation of VEGFR2 expression. Further, the cornea in PD-L1 knockout mice induces higher level of angiogenic responses than wild type, via CD80 not PD-1 (130), suggesting that the ratio of CD80 and PD-1 expression on the recruited immune cells could determine either angiogenesis or immune deviation (Figure 4B). CD80, known as B7.1, is expressed on antigen presenting cells such as monocytes. In addition, VEGF treatment induces PD-L1 in tumors (131). Taken together, PD-L1 is essential in ocular immunobiology and its molecular signaling pathway could be part of the NV process. Further understanding of PD-1/PD-L1 in retinal and choroidal NV should be pursued and compared with cancer studies.
Inflammatory pathways in retinal and choroidal NV: IGF system
The IGF system consists of IGF-I and IGF-II ligands and their cognate receptors, IGF-binding proteins (IGFBPs), and IGFBP-specific proteases, all regulating cell growth and differentiation. IGF-I and IGF-I receptor (IGF-IR) are detected in retinal ECs, RPE and Müller cells (132-134) (Figure 4C). IGF-I is considered one of pro-angiogenic factors in PDR (135) and Wet AMD (136). In PDR, vitreous IGF-I levels are elevated (137-139), and genetic (140) and injected overexpression (139,141,142) of intravitreal IGF-l results in BRB breakdown, VEGF upregulation, loss of vascular integrity and retinal NV. On the other hand, IGF-lR inhibitors decrease VEGF secretion and retinal and choroidal NV (143,144).
IGFBP-3 is a major IGFBP species in circulation and its binding to IGF-I decreases IGF-I bioactivity by preventing IGF-1 binding to IGF-IR (145). IGFBP-3 further exerts IGF/IGF-R-independent cellular functions, including anti-inflammation, either pro- or anti-apoptosis and DNA damage repair (145-147). Interestingly, obese populations display a decrease in functional IGFBP-3 levels and an increase in proteolytic IGFBP-3 fragments in circulation (148,149). Cell and animal studies have shown the protective role of IGFBP-3 in retinal NV. The restoration of intravitreal IGFBP-3 results in protection of ischemic retinal injury and DR in murine models (150,151). IGFBP-3 reduces retina vascular permeability, inhibits pro-inflammation and protects from retinal EC apoptosis (152-155). IGFBP-3 further recruits bone marrow-derived cells, vascular progenitors and hematopoietic stem cells to sites of retinal hypoxia and ischemic injury, and stabilizes mural cells, maintains endothelial integrity, and reduces inflammation (150,154). Taken together, increased IGF-I and nonfunctional IGFBP-3 fragments seem to be early players in retinal and choroidal NV formation.
Inflammatory pathways in retinal and choroidal NV: sphigosine-1-phosphate (S1P)/S1P receptors (S1PRs)
S1P is a sphingosine-containing bioactive lipid generated from ceramide, mainly secreted by RBCs, ECs and activated platelets. It binds five G protein-coupled surface receptors (S1PRs1-5). S1PRs are found on retinal ganglion cells, RPE, ECs, astrocytes, microglia, monocytes, dendric cells and lymphocytes (156-159). The function of S1P/S1PRs is not fully understood, but they are associated with vascular barrier function and inflammatory responses (159). Increased level of S1P is observed at inflammation sites and recruits immune cells. SIP/SIPRs signaling also induces NLRP3 activation (158), and triggers M1 and Th1-polarized proinflammatory responses (160,161). However, the S1P/S1PRs axis also activates negative feedback, reducing vascular leakage (162), as well. In retina (Figure 4D), RPE is considered a major source of S1P during choroidal NV progression (163), and astrocytes and Müller glia might be other sources of S1P during retinal NV. Photoreceptors also express S1P in response to light damage (159,164,165), and S1P is generated by sphingosine kinases that are upregulated by HIF1α (166). Hypoxia upregulates S1P/S1PRs axis, subsequently generates nitric oxide and increases vasodilation of ECs (167). Since S1PR modulator, FTY720 (fingolimod, S1PR agonist) was approved for multiple sclerosis in 2010 (168,169), fingolimod and other S1P/S1PR modulators are under clinical trial for brain diseases such as acute stroke, amyotrophic lateral sclerosis, schizophrenia, Rett syndrome, glioblastoma and other autoimmune and inflammatory diseases, like psoriasis and Crohn’s (170). The modulation of S1P/S1PR using anti-S1P antibody (Sonepcizumab, S1P antagonist) suppresses retinal and choroidal NV by suppressing inflammation and reducing the recruited microglia and macrophages in animals (166). However, Sonepcizumab did not show a statistically significant improvement in the visual acuity of Wet AMD patients, as a monotherapy and adjunctive to anti-VEGF agents in the Phase 2 clinical trial (166,171). Since S1P and complex signaling of S1P/S1PRs1-5 have a dual role in inflammatory responses (170), the cellular and molecular knowledge of SIP and S1PRs in retina diseases might provide a possible intervention strategy for retina inflammatory diseases. Of note, S1PR1 expression is upregulated by galectin-1 (Gal-1) and overexpressed Gal-1 and SIP/S1PRs are known to be associated with epithelial to mesenchymal transition (EMT) in cancer (172). EMT is a process where epithelial cells lose polarity and adhesion but obtain migratory and differentiated properties. These similar mechanisms might be associated with RPE inward migration, causing broken outer BRB.
Conclusions
We reviewed cells and inflammatory pathways associated with retinal and choroidal NV in DR and AMD here to provide the current available knowledge and insight in their therapeutic targets. Therapy of anti-VEGF agents for ocular NV diseases has offered substantial improvement in outcomes (173-175), but therapeutic innovation is still a requisite, as approximately up to 40–50% of both Wet AMD (163,176) and PDR (177,178) patients do not fully respond to anti-VEGF therapy. Given that the pathogenesis and pathobiology of DR and AMD has yet to be clearly elucidated, a deeper understanding of unbalanced inflammation will warrant a continued effort in expanding our knowledge. Furthermore, additional studies may complement current anti-VEGF therapeutic approaches and pave the way for next generation therapeutics to treat chronic retinal and choroidal NV.
Acknowledgments
We thank Drs. Gail Seabold (National Institutes of Health, USA), DongHyun Jo (Department of Anatomy and Cell biology, Seoul National University College of Medicine, South Korea) and Qingguo Xu (Department of Pharmaceutics, Virginia Commonwealth University, USA) for a critical reading of the manuscript and helpful comments.
Funding: This work was supported, in part, by VCU Quest for Innovation Commercialization Fund and Intellectual Property Foundation Support Fund (YO).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/aes-21-4
Peer Review File: Available at https://dx.doi.org/10.21037/aes-21-4
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aes-21-4). The authors have no financial conflicts of interest to declare, however, the authors (YO, SYK) have a VCU provisional patent application (USPPA62/904,117, Sept 23, 2020) relating to the IGFBP3/TEMEM219 modulation for ocular therapies. The author (SYK) was an employee of ExosomePlus, Inc. (South Korea). The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wong-Riley MT. Energy metabolism of the visual system. Eye Brain 2010;2:99-116. [Crossref] [PubMed]
- Logsdon EA, Finley SD, Popel AS, et al. A systems biology view of blood vessel growth and remodelling. J Cell Mol Med 2014;18:1491-508. [Crossref] [PubMed]
- Key facts of Diabetes, World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes
- Kempen JH, O'Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004;122:552-63. [Crossref] [PubMed]
- Roy MS, Klein R, O'Colmain BJ, et al. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol 2004;122:546-51. [Crossref] [PubMed]
- Kuwabara T, Cogan DG. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol 1963;69:492-502. [Crossref] [PubMed]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 2005;97:512-23. [Crossref] [PubMed]
- Hammes HP, Lin J, Renner O, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002;51:3107-12. [Crossref] [PubMed]
- COGAN DG. TOUSSAINT D, KUWABARA T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 1961;66:366-78. [Crossref] [PubMed]
- Li W, Yanoff M, Liu X, et al. Retinal capillary pericyte apoptosis in early human diabetic retinopathy. Chin Med J (Engl) 1997;110:659-63. [PubMed]
- Arboleda-Velasquez JF, Valdez CN, Marko CK, et al. From pathobiology to the targeting of pericytes for the treatment of diabetic retinopathy. Curr Diab Rep 2015;15:573. [Crossref] [PubMed]
- Condren AB, Kumar A, Mettu P, et al. Perivascular mural cells of the mouse choroid demonstrate morphological diversity that is correlated to vasoregulatory function. PLoS One 2013;8:e53386 [Crossref] [PubMed]
- Allende A, Madigan MC, Provis JM. Endothelial cell proliferation in the choriocapillaris during human retinal differentiation. Br J Ophthalmol 2006;90:1046-51. [Crossref] [PubMed]
- Bonilha VL. Age and disease-related structural changes in the retinal pigment epithelium. Clin Ophthalmol 2008;2:413-24. [Crossref] [PubMed]
- Kim SY. Retinal phagocytes in age-related macular degeneration. Macrophage (Houst) 2015;2:e698 [PubMed]
- Ponnalagu M, Subramani M, Jayadev C, et al. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine 2017;95:126-35. [Crossref] [PubMed]
- Simó R, Villarroel M, Corraliza L, et al. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol 2010;2010:190724 [Crossref] [PubMed]
- Samuels IS, Bell BA, Pereira A, et al. Early retinal pigment epithelium dysfunction is concomitant with hyperglycemia in mouse models of type 1 and type 2 diabetes. J Neurophysiol 2015;113:1085-99. [Crossref] [PubMed]
- Martin G, Schlunck G, Hansen LL, et al. Differential expression of angioregulatory factors in normal and CNV-derived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 2004;242:321-6. [Crossref] [PubMed]
- Wang X, Ma W, Han S, et al. TGF-β participates choroid neovascularization through Smad2/3-VEGF/TNF-α signaling in mice with Laser-induced wet age-related macular degeneration. Sci Rep 2017;7:9672. [Crossref] [PubMed]
- Jiang J, Xu K, Wang L, et al. Pharmacology study of a chimeric decoy receptor trap fusion protein on retina neovascularization by dual blockage of VEGF and FGF-2. Eur J Pharm Sci 2018;121:251-9. [Crossref] [PubMed]
- Stahl A, Paschek L, Martin G, et al. Combinatory inhibition of VEGF and FGF2 is superior to solitary VEGF inhibition in an in vitro model of RPE-induced angiogenesis. Graefes Arch Clin Exp Ophthalmol 2009;247:767-73. [Crossref] [PubMed]
- Recalde S, Zarranz-Ventura J, Fernández-Robredo P, et al. Transforming growth factor-β inhibition decreases diode laser-induced choroidal neovascularization development in rats: P17 and P144 peptides. Invest Ophthalmol Vis Sci 2011;52:7090-7. [Crossref] [PubMed]
- Ma W, Silverman SM, Zhao L, et al. Absence of TGFβ signaling in retinal microglia induces retinal degeneration and exacerbates choroidal neovascularization. Elife 2019;8:42049. [Crossref]
- Schlecht A, Leimbeck SV, Jägle H, et al. Deletion of Endothelial Transforming Growth Factor-β Signaling Leads to Choroidal Neovascularization. Am J Pathol 2017;187:2570-89. [Crossref] [PubMed]
- Ravera V, Giani A, Pellegrini M, et al. Comparison among different diagnostic methods in the study of type and activity of choroidal neovascular membranes in age-related macular degeneration. Retina 2019;39:281-7. [Crossref] [PubMed]
- Li R, Wen R, Banzon T, et al. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS One 2011;6:e23148 [Crossref] [PubMed]
- Sonoda S, Sreekumar PG, Kase S, et al. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany NY) 2009;2:28-42. [Crossref] [PubMed]
- Kay P, Yang YC, Paraoan L. Directional protein secretion by the retinal pigment epithelium: roles in retinal health and the development of age-related macular degeneration. J Cell Mol Med 2013;17:833-43. [Crossref] [PubMed]
- Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci 2006;47:3135-42. [Crossref] [PubMed]
- Yi X, Mai LC, Uyama M, et al. Time-course expression of vascular endothelial growth factor as related to the development of the retinochoroidal vasculature in rats. Exp Brain Res 1998;118:155-60. [Crossref] [PubMed]
- Adamis AP, Shima DT, Yeo KT, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun 1993;193:631-8. [Crossref] [PubMed]
- Holtkamp GM, De Vos AF, Peek R, et al. Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells. Clin Exp Immunol 1999;118:35-40. [Crossref] [PubMed]
- Holtkamp GM, Van Rossem M, de Vos AF, et al. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol 1998;112:34-43. [Crossref] [PubMed]
- Feit-Leichman RA, Kinouchi R, Takeda M, et al. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci 2005;46:4281-7. [Crossref] [PubMed]
- Rungger-Brändle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci 2000;41:1971-80. [PubMed]
- Luna G, Keeley PW, Reese BE, et al. Astrocyte structural reactivity and plasticity in models of retinal detachment. Exp Eye Res 2016;150:4-21. [Crossref] [PubMed]
- Edwards MM, McLeod DS, Bhutto IA, et al. Idiopathic preretinal glia in aging and age-related macular degeneration. Exp Eye Res 2016;150:44-61. [Crossref] [PubMed]
- Kaur C, Sivakumar V, Foulds WS. Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci 2006;47:1126-41. [Crossref] [PubMed]
- Bai Y, Ma JX, Guo J, et al. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol 2009;219:446-54. [Crossref] [PubMed]
- Edwards MM, McLeod DS, Bhutto IA, et al. Subretinal Glial Membranes in Eyes With Geographic Atrophy. Invest Ophthalmol Vis Sci 2017;58:1352-67. [Crossref] [PubMed]
- Kim SY, Kambhampati SP, Bhutto IA, et al. Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model. Exp Eye Res 2021;203:108391 [Crossref] [PubMed]
- Karlstetter M, Scholz R, Rutar M, et al. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 2015;45:30-57. [Crossref] [PubMed]
- Grigsby JG, Cardona SM, Pouw CE, et al. The role of microglia in diabetic retinopathy. J Ophthalmol 2014;2014:705783 [Crossref] [PubMed]
- Checchin D, Sennlaub F, Levavasseur E, et al. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci 2006;47:3595-602. [Crossref] [PubMed]
- Rathnasamy G, Foulds WS, Ling EA, et al. Retinal microglia - A key player in healthy and diseased retina. Prog Neurobiol 2019;173:18-40. [Crossref] [PubMed]
- Rymo SF, Gerhardt H, Wolfhagen Sand F, et al. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One 2011;6:e15846 [Crossref] [PubMed]
- Shi YY, Wang YS, Zhang ZX, et al. Monocyte/macrophages promote vasculogenesis in choroidal neovascularization in mice by stimulating SDF-1 expression in RPE cells. Graefes Arch Clin Exp Ophthalmol 2011;249:1667-79. [Crossref] [PubMed]
- Yamada K, Sakurai E, Itaya M, et al. Inhibition of laser-induced choroidal neovascularization by atorvastatin by downregulation of monocyte chemotactic protein-1 synthesis in mice. Invest Ophthalmol Vis Sci 2007;48:1839-43. [Crossref] [PubMed]
- Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol 2004;122:1013-8. [Crossref] [PubMed]
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis 2012;33:949-55. [Crossref] [PubMed]
- Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res 2016;4:83-91. [Crossref] [PubMed]
- Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14-20. [Crossref] [PubMed]
- Fukushi J, Ono M, Morikawa W, et al. The activity of soluble VCAM-1 in angiogenesis stimulated by IL-4 and IL-13. J Immunol 2000;165:2818-23. [Crossref] [PubMed]
- Hong KH, Cho ML, Min SY, et al. Effect of interleukin-4 on vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Clin Exp Immunol 2007;147:573-9. [Crossref] [PubMed]
- Volpert OV, Fong T, Koch AE, et al. Inhibition of angiogenesis by interleukin 4. J Exp Med 1998;188:1039-46. [Crossref] [PubMed]
- Jetten N, Verbruggen S, Gijbels MJ, et al. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014;17:109-18. [Crossref] [PubMed]
- Parisi L, Gini E, Baci D, et al. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J Immunol Res 2018;2018:8917804 [Crossref] [PubMed]
- Seignez C, Phillipson M. The multitasking neutrophils and their involvement in angiogenesis. Curr Opin Hematol 2017;24:3-8. [Crossref] [PubMed]
- Bouhlel MA, Derudas B, Rigamonti E, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 2007;6:137-43. [Crossref] [PubMed]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007;447:1116-20. [Crossref] [PubMed]
- Hevener AL, Olefsky JM, Reichart D, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 2007;117:1658-69. [Crossref] [PubMed]
- Lechner J, Chen M, Hogg RE, et al. Alterations in Circulating Immune Cells in Neovascular Age-Related Macular Degeneration. Sci Rep 2015;5:16754. [Crossref] [PubMed]
- Krogh Nielsen M, Hector SM, Allen K, et al. Altered activation state of circulating neutrophils in patients with neovascular age-related macular degeneration. Immun Ageing 2017;14:18. [Crossref] [PubMed]
- Krause TA, Alex AF, Engel DR, et al. VEGF-production by CCR2-dependent macrophages contributes to laser-induced choroidal neovascularization. PLoS One 2014;9:e94313 [Crossref] [PubMed]
- Tsutsumi-Miyahara C, Sonoda KH, Egashira K, et al. The relative contributions of each subset of ocular infiltrated cells in experimental choroidal neovascularisation. Br J Ophthalmol 2004;88:1217-22. [Crossref] [PubMed]
- Zhou Y, Sheets KG, Knott EJ, et al. Cellular and 3D optical coherence tomography assessment during the initiation and progression of retinal degeneration in the Ccl2/Cx3cr1-deficient mouse. Exp Eye Res 2011;93:636-48. [Crossref] [PubMed]
- Sennlaub F, Auvynet C, Calippe B, et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med 2013;5:1775-93. [Crossref] [PubMed]
- Tuo J, Bojanowski CM, Zhou M, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci 2007;48:3827-36. [Crossref] [PubMed]
- Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med 2003;9:1390-7. [Crossref] [PubMed]
- Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol 2016;787:94-104. [Crossref] [PubMed]
- Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 2010;29:95-112. [Crossref] [PubMed]
- Atchison E, Barkmeier A. The Role of Systemic Risk Factors in Diabetic Retinopathy. Curr Ophthalmol Rep 2016;4:84-9. [Crossref] [PubMed]
- Wat N, Wong RL, Wong IY. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med J 2016;22:589-99. [Crossref] [PubMed]
- Cheung CM, Wong TY. Is age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatment. J Intern Med 2014;276:140-53. [Crossref] [PubMed]
- Dasari B, Prasanthi JR, Marwarha G, et al. Cholesterol-enriched diet causes age-related macular degeneration-like pathology in rabbit retina. BMC Ophthalmol 2011;11:22. [Crossref] [PubMed]
- Wang J, Yang MM, Li YB, et al. Association of CFH and CFB gene polymorphisms with retinopathy in type 2 diabetic patients. Mediators Inflamm 2013;2013:748435 [Crossref] [PubMed]
- Yang MM, Wang J, Ren H, et al. Genetic Investigation of Complement Pathway Genes in Type 2 Diabetic Retinopathy: An Inflammatory Perspective. Mediators Inflamm 2016;2016:1313027 [Crossref] [PubMed]
- Fritsche LG, Fariss RN, Stambolian D, et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet 2014;15:151-71. [Crossref] [PubMed]
- Ricklin D, Hajishengallis G, Yang K, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 2010;11:785-97. [Crossref] [PubMed]
- Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev 2008;223:300-16. [Crossref] [PubMed]
- Clark SJ, Bishop PN. The eye as a complement dysregulation hotspot. Semin Immunopathol 2018;40:65-74. [Crossref] [PubMed]
- Gao X, Liu H, He B, et al. Resistance to Streptozotocin-Induced Autoimmune Diabetes in Absence of Complement C3: Myeloid-Derived Suppressor Cells Play a Role. PLoS One 2013;8:e66334 [Crossref] [PubMed]
- Xu D, Yi H, Yu S, et al. Association of Complement C5 Gene Polymorphisms with Proliferative Diabetic Retinopathy of Type 2 Diabetes in a Chinese Han Population. PLoS One 2016;11:e0149704 [Crossref] [PubMed]
- Tan X, Fujiu K, Manabe I, et al. Choroidal neovascularization is inhibited via an intraocular decrease of inflammatory cells in mice lacking complement component C3. Sci Rep 2015;5:15702. [Crossref] [PubMed]
- Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A 2006;103:2328-33. [Crossref] [PubMed]
- Yu M, Zou W, Peachey NS, et al. A novel role of complement in retinal degeneration. Invest Ophthalmol Vis Sci 2012;53:7684-92. [Crossref] [PubMed]
- Wu J, Sun X. Complement system and age-related macular degeneration: drugs and challenges. Drug Des Devel Ther 2019;13:2413-25. [Crossref] [PubMed]
- Holz FG, Sadda SR, Busbee B, et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol 2018;136:666-77. [Crossref] [PubMed]
- Jaffe GJ, Westby K, Csaky KG, et al. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021;128:576-86. [Crossref] [PubMed]
- Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020;127:186-95. [Crossref] [PubMed]
- Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol 2006;13:175-81. [Crossref] [PubMed]
- Federsppiel B, Melhado IG, Duncan AM, et al. Molecular cloning of the cDNA and chromosomal localization of the gene for a putative seven-transmembrane segment (7-TMS) receptor isolated from human spleen. Genomics 1993;16:707-12. [Crossref] [PubMed]
- Nomura H, Nielsen BW, Matsushima K. Molecular cloning of cDNAs encoding a LD78 receptor and putative leukocyte chemotactic peptide receptors. Int Immunol 1993;5:1239-49. [Crossref] [PubMed]
- Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 1996;382:833-5. [Crossref] [PubMed]
- Zhang ZX, Wang YS, Shi YY, et al. Hypoxia specific SDF-1 expression by retinal pigment epithelium initiates bone marrow-derived cells to participate in Choroidal neovascularization in a laser-induced mouse model. Curr Eye Res 2011;36:838-49. [Crossref] [PubMed]
- Lima e Silva R, Shen J, Hackett SF, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J 2007;21:3219-30. [Crossref] [PubMed]
- Lee E, Rewolinski D. Evaluation of CXCR4 inhibition in the prevention and intervention model of laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci 2010;51:3666-72. [Crossref] [PubMed]
- Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015;21:677-87. [Crossref] [PubMed]
- Gao J, Liu RT, Cao S, et al. NLRP3 inflammasome: activation and regulation in age-related macular degeneration. Mediators Inflamm 2015;2015:690243 [Crossref] [PubMed]
- Lee HM, Kim JJ, Kim HJ, et al. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013;62:194-204. [Crossref] [PubMed]
- Zhang X, Dai J, Li L, et al. NLRP3 Inflammasome Expression and Signaling in Human Diabetic Wounds and in High Glucose Induced Macrophages. J Diabetes Res 2017;2017:5281358 [Crossref] [PubMed]
- Qiu YY, Tang LQ. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol Res 2016;114:251-64. [Crossref] [PubMed]
- El-Horany HE, Abd-Ellatif RN, Watany M, et al. NLRP3 expression and urinary HSP72 in relation to biomarkers of inflammation and oxidative stress in diabetic nephropathy patients. IUBMB Life 2017;69:623-30. [Crossref] [PubMed]
- Loukovaara S, Piippo N, Kinnunen K, et al. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol 2017;95:803-8. [Crossref] [PubMed]
- Chen H, Zhang X, Liao N, et al. Enhanced Expression of NLRP3 Inflammasome-Related Inflammation in Diabetic Retinopathy. Invest Ophthalmol Vis Sci 2018;59:978-85. [Crossref] [PubMed]
- Chaurasia SS, Lim RR, Parikh BH, et al. The NLRP3 Inflammasome May Contribute to Pathologic Neovascularization in the Advanced Stages of Diabetic Retinopathy. Sci Rep 2018;8:2847. [Crossref] [PubMed]
- Wang S, Li Y, Fan J, et al. Interleukin-22 ameliorated renal injury and fibrosis in diabetic nephropathy through inhibition of NLRP3 inflammasome activation. Cell Death Dis 2017;8:e2937 [Crossref] [PubMed]
- Lu M, Yin N, Liu W, et al. Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signaling. Biomed Res Int 2017;2017:1516985 [Crossref] [PubMed]
- Samra YA, Said HS, Elsherbiny NM, et al. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: role of NF-κB and NLRP3 inflammasome. Life Sci 2016;157:187-99. [Crossref] [PubMed]
- Liu Q, Zhang F, Zhang X, et al. Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol Cell Biochem 2018;445:105-15. [Crossref] [PubMed]
- Sui A, Chen X, Shen J, et al. Inhibiting the NLRP3 inflammasome with MCC950 ameliorates retinal neovascularization and leakage by reversing the IL-1β/IL-18 activation pattern in an oxygen-induced ischemic retinopathy mouse model. Cell Death Dis 2020;11:901. [Crossref] [PubMed]
- Marneros AG. NLRP3 inflammasome blockade inhibits VEGF-A-induced age-related macular degeneration. Cell Rep 2013;4:945-58. [Crossref] [PubMed]
- Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med 2012;18:791-8. [Crossref] [PubMed]
- Fowler BJ, Gelfand BD, Kim Y, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 2014;346:1000-3. [Crossref] [PubMed]
- Liu RT, Gao J, Cao S, et al. Inflammatory mediators induced by amyloid-beta in the retina and RPE in vivo: implications for inflammasome activation in age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:2225-37. [Crossref] [PubMed]
- Hu W, Jiang A, Liang J, et al. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest Ophthalmol Vis Sci 2008;49:407-15. [Crossref] [PubMed]
- Wu H, Hwang DK, Song X, et al. Association between Aqueous Cytokines and Diabetic Retinopathy Stage. J Ophthalmol 2017;2017:9402198 [Crossref] [PubMed]
- Doyle SL, Ozaki E, Brennan K, et al. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci Transl Med 2014;6:230ra44 [Crossref] [PubMed]
- Ijima R, Kaneko H, Ye F, et al. Interleukin-18 induces retinal pigment epithelium degeneration in mice. Invest Ophthalmol Vis Sci 2014;55:6673-8. [Crossref] [PubMed]
- Tseng WA, Thein T, Kinnunen K, et al. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:110-20. [Crossref] [PubMed]
- Carmo A, Cunha-Vaz JG, Carvalho AP, et al. Effect of cyclosporin-A on the blood--retinal barrier permeability in streptozotocin-induced diabetes. Mediators Inflamm 2000;9:243-8. [Crossref] [PubMed]
- Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci 2004;45:4161-6. [Crossref] [PubMed]
- Abe T, Sugano E, Saigo Y, et al. Interleukin-1beta and barrier function of retinal pigment epithelial cells (ARPE-19): aberrant expression of junctional complex molecules. Invest Ophthalmol Vis Sci 2003;44:4097-104. [Crossref] [PubMed]
- Shi G, Chen S, Wandu WS, et al. Inflammasomes Induced by 7-Ketocholesterol and Other Stimuli in RPE and in Bone Marrow-Derived Cells Differ Markedly in Their Production of IL-1β and IL-18. Invest Ophthalmol Vis Sci 2015;56:1658-64. [Crossref] [PubMed]
- Hirano Y, Yasuma T, Mizutani T, et al. IL-18 is not therapeutic for neovascular age-related macular degeneration. Nat Med 2014;20:1372-5. [Crossref] [PubMed]
- Shen J, Choy DF, Yoshida T, et al. Interleukin-18 has antipermeablity and antiangiogenic activities in the eye: reciprocal suppression with VEGF. J Cell Physiol 2014;229:974-83. [Crossref] [PubMed]
- Yang W, Li H, Chen PW, et al. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest Ophthalmol Vis Sci 2009;50:273-80. [Crossref] [PubMed]
- Shen L, Jin Y, Freeman GJ, et al. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol 2007;179:3672-9. [Crossref] [PubMed]
- Jin Y, Chauhan SK, El Annan J, et al. A novel function for programmed death ligand-1 regulation of angiogenesis. Am J Pathol 2011;178:1922-9. [Crossref] [PubMed]
- Yi M, Jiao D, Qin S, et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer 2019;18:60. [Crossref] [PubMed]
- Rosenthal R, Wohlleben H, Malek G, et al. Insulin-like growth factor-1 contributes to neovascularization in age-related macular degeneration. Biochem Biophys Res Commun 2004;323:1203-8. [Crossref] [PubMed]
- Li F, Cao W, Steinberg RH, et al. Basic FGF-induced down-regulation of IGF-I mRNA in cultured rat Müller cells. Exp Eye Res 1999;68:19-27. [Crossref] [PubMed]
- Shaw LC, Pan H, Afzal A, et al. Proliferating endothelial cell-specific expression of IGF-I receptor ribozyme inhibits retinal neovascularization. Gene Ther 2006;13:752-60. [Crossref] [PubMed]
- Grant MB, Afzal A, Spoerri P, et al. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs 2004;13:1275-93. [Crossref] [PubMed]
- Lambooij AC, van Wely KH, Lindenbergh-Kortleve DJ, et al. Insulin-like growth factor-I and its receptor in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2003;44:2192-8. [Crossref] [PubMed]
- Grant M, Russell B, Fitzgerald C, et al. Insulin-like growth factors in vitreous. Studies in control and diabetic subjects with neovascularization. Diabetes 1986;35:416-20. [Crossref] [PubMed]
- Dills DG, Moss SE, Klein R, et al. Association of elevated IGF-I levels with increased retinopathy in late-onset diabetes. Diabetes 1991;40:1725-30. [Crossref] [PubMed]
- Haurigot V, Villacampa P, Ribera A, et al. Increased intraocular insulin-like growth factor-I triggers blood-retinal barrier breakdown. J Biol Chem 2009;284:22961-9. [Crossref] [PubMed]
- Ruberte J, Ayuso E, Navarro M, et al. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J Clin Invest 2004;113:1149-57. [Crossref] [PubMed]
- Danis RP, Bingaman DP. Insulin-like growth factor-1 retinal microangiopathy in the pig eye. Ophthalmology 1997;104:1661-9. [Crossref] [PubMed]
- Grant MB, Mames RN, Fitzgerald C, et al. Insulin-like growth factor I acts as an angiogenic agent in rabbit cornea and retina: comparative studies with basic fibroblast growth factor. Diabetologia 1993;36:282-91. [Crossref] [PubMed]
- Hernández C, Simó R. Strategies for blocking angiogenesis in diabetic retinopathy: from basic science to clinical practice. Expert Opin Investig Drugs 2007;16:1209-26. [Crossref] [PubMed]
- Economou MA, Wu J, Vasilcanu D, et al. Inhibition of VEGF secretion and experimental choroidal neovascularization by picropodophyllin (PPP), an inhibitor of the insulin-like growth factor-1 receptor. Invest Ophthalmol Vis Sci 2008;49:2620-6. [Crossref] [PubMed]
- Cai Q, Dozmorov M, Oh Y. IGFBP-3/IGFBP-3 Receptor System as an Anti-Tumor and Anti-Metastatic Signaling in Cancer. Cells 2020;9:1261. [Crossref] [PubMed]
- Baxter RC. Nuclear actions of insulin-like growth factor binding protein-3. Gene 2015;569:7-13. [Crossref] [PubMed]
- Baxter RC. Insulin-like growth factor binding protein-3 (IGFBP-3): Novel ligands mediate unexpected functions. J Cell Commun Signal 2013;7:179-89. [Crossref] [PubMed]
- Mohanraj L, Kim HS, Li W, et al. IGFBP-3 inhibits cytokine-induced insulin resistance and early manifestations of atherosclerosis. PLoS One 2013;8:e55084 [Crossref] [PubMed]
- Robins JL, Cai Q, Oh Y. The impact of neutrophil proteinase 3 on IGFBP-3 proteolysis in obesity. Intern Med 2014;S6:003. doi:
10.4172/2165-8048.S6-003 .10.4172/2165-8048.S6-003 - Lofqvist C, Chen J, Connor KM, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci U S A 2007;104:10589-94. [Crossref] [PubMed]
- Jiang Y, Zhang Q, Steinle JJ. Intravitreal injection of IGFBP-3 restores normal insulin signaling in diabetic rat retina. PLoS One 2014;9:e93788 [Crossref] [PubMed]
- Jiang Y, Pagadala J, Miller DD, et al. Insulin-like growth factor-1 binding protein 3 (IGFBP-3) promotes recovery from trauma-induced expression of inflammatory and apoptotic factors in retina. Cytokine 2014;70:115-9. [Crossref] [PubMed]
- Zhang Q, Jiang Y, Steinle JJ. IGFBP-3 reduces eNOS and PKCzeta phosphorylation, leading to lowered VEGF levels. Mol Vis 2015;21:604-11. [PubMed]
- Nguyen DV, Li Calzi S, Shaw LC, et al. An ocular view of the IGF-IGFBP system. Growth Horm IGF Res 2013;23:45-52. [Crossref] [PubMed]
- Kielczewski JL, Li Calzi S, Shaw LC, et al. Free insulin-like growth factor binding protein-3 (IGFBP-3) reduces retinal vascular permeability in association with a reduction of acid sphingomyelinase (ASMase). Invest Ophthalmol Vis Sci 2011;52:8278-86. [Crossref] [PubMed]
- Rothhammer V, Kenison JE, Tjon E, et al. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci U S A 2017;114:2012-7. [Crossref] [PubMed]
- Karuppuchamy T, Behrens EH, González-Cabrera P, et al. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol 2017;10:162-71. [Crossref] [PubMed]
- Chen Z, Doyle TM, Luongo L, et al. Sphingosine-1-phosphate receptor 1 activation in astrocytes contributes to neuropathic pain. Proc Natl Acad Sci U S A 2019;116:10557-62. [Crossref] [PubMed]
- Simón MV, Prado Spalm FH, Vera MS, et al. Sphingolipids as Emerging Mediators in Retina Degeneration. Front Cell Neurosci 2019;13:246. [Crossref] [PubMed]
- Gaire BP, Song MR, Choi JW. Sphingosine 1-phosphate receptor subtype 3 (S1P3) contributes to brain injury after transient focal cerebral ischemia via modulating microglial activation and their M1 polarization. J Neuroinflammation 2018;15:284. [Crossref] [PubMed]
- Yang J, Yang L, Tian L, et al. Sphingosine 1-Phosphate (S1P)/S1P Receptor2/3 Axis Promotes Inflammatory M1 Polarization of Bone Marrow-Derived Monocyte/Macrophage via G(α)i/o/PI3K/JNK Pathway. Cell Physiol Biochem 2018;49:1677-93. [Crossref] [PubMed]
- Danese S, Furfaro F, Vetrano S. Targeting S1P in Inflammatory Bowel Disease: New Avenues for Modulating Intestinal Leukocyte Migration. J Crohns Colitis 2018;12:S678-86. [Crossref] [PubMed]
- CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-908. [Crossref] [PubMed]
- Obinata H, Hla T. Sphingosine 1-phosphate and inflammation. Int Immunol 2019;31:617-25. [Crossref] [PubMed]
- Terao R, Honjo M, Ueta T, et al. Light Stress-Induced Increase of Sphingosine 1-Phosphate in Photoreceptors and Its Relevance to Retinal Degeneration. Int J Mol Sci 2019;20:3670. [Crossref] [PubMed]
- Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol 2011;162:1225-38. [Crossref] [PubMed]
- Alganga H, Almabrouk TAM, Katwan OJ, et al. Short Periods of Hypoxia Upregulate Sphingosine Kinase 1 and Increase Vasodilation of Arteries to Sphingosine 1-Phosphate (S1P) via S1P3. J Pharmacol Exp Ther 2019;371:63-74. [Crossref] [PubMed]
- Strader CR, Pearce CJ, Oberlies NH. Fingolimod (FTY720): a recently approved multiple sclerosis drug based on a fungal secondary metabolite. J Nat Prod 2011;74:900-7. [Crossref] [PubMed]
- Gajofatto A, Turatti M, Monaco S, et al. Clinical efficacy, safety, and tolerability of fingolimod for the treatment of relapsing-remitting multiple sclerosis. Drug Healthc Patient Saf 2015;7:157-67. [Crossref] [PubMed]
- Park SJ, Im DS. Sphingosine 1-Phosphate Receptor Modulators and Drug Discovery. Biomol Ther (Seoul) 2017;25:80-90. [Crossref] [PubMed]
- Hanout M, Ferraz D, Ansari M, et al. Therapies for neovascular age-related macular degeneration: current approaches and pharmacologic agents in development. Biomed Res Int 2013;2013:830837 [Crossref] [PubMed]
- You X, Wang Y, Wu J, et al. Galectin-1 Promotes Metastasis in Gastric Cancer Through a Sphingosine-1-Phosphate Receptor 1-Dependent Mechanism. Cell Physiol Biochem 2018;51:11-30. [Crossref] [PubMed]
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-31. [Crossref] [PubMed]
- Kaszubski P, Ben Ami T, Saade C, et al. Geographic Atrophy and Choroidal Neovascularization in the Same Eye: A Review. Ophthalmic Res 2016;55:185-93. [Crossref] [PubMed]
- Tolentino MS, Tolentino AJ, Tolentino MJ. Current and investigational drugs for the treatment of diabetic retinopathy. Expert Opin Investig Drugs 2016;25:1011-22. [Crossref] [PubMed]
- Cunnusamy K, Ufret-Vincenty R, Wang S. Next-generation therapeutic solutions for age-related macular degeneration. Pharm Pat Anal 2012;1:193-206. [Crossref] [PubMed]
- Bressler SB, Qin H, Melia M, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol 2013;131:1033-40. [Crossref] [PubMed]
- Ip MS, Domalpally A, Hopkins JJ, et al. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol 2012;130:1145-52. [Crossref] [PubMed]
Cite this article as: Kim SY, Kim Y, Oh Y. Inflammatory pathways in pathological neovascularization in retina and choroid: a narrative review on the inflammatory drug target molecules in retinal and choroidal neovascularization. Ann Eye Sci 2021;6:24.


