
Narrative review of risuteganib for the treatment of dry age-related macular degeneration (AMD)
General age-related macular degeneration (AMD)
AMD is a leading cause of blindness in developed countries and accounts for 8.7% of blindness worldwide (1). AMD is characterized by degeneration of the retinal pigment epithelium (RPE) and neurosensory retina. This degeneration leads to irreversible vision loss and most commonly effects older individuals. There are two forms of AMD, non-exudative (dry) and exudative (wet). Dry AMD is more prevalent, affecting 85–90% of patients suffering from AMD (2). Exudative AMD is characterized by neovascularization and fluid accumulation within the macula, whereas non-exudative AMD lacks neovascularization. While several treatments exist for the treatment of exudative AMD, there are limited treatment options of non-exudative AMD. Existing recommendations for dry AMD consist of lifestyle modifications and micronutrient supplementation (3,4). While this review will focus on integrin inhibition, several interventions targeting the complement pathway, neuropeptides, mitochondrial protective factors and both induced pluripotent and embryologic stem cells are under investigation and summarized in Table 1. We present the following article in accordance with Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/aes-21-12).
Table 1
| Category | Drug | Mechanism of action | Dry AMD/geographic atrophy clinical trials |
|---|---|---|---|
| Integrin inhibitor | Risuteganib; Luminate (Alg-1001) | Inhibits integrin heterodimers αVβ3, αVβ5, α5β1, and αMβ2 | NCT03626636 (2018) phase 2 |
| Complement pathway inhibitor | Zimura (ARC-1905) | Inhibits complement C5 | NCT00950638 (2009) phase 1; NCT02686658 (2016) phase 2 |
| Complement pathway inhibitor | Lampalizumab | Inhibits complement factor D | NCT02288559 (2014) phase 2; NCT01602120 (2012) phase 2; NCT02247531 (2014) phase 3; NCT02247479 phase 3 (2014); NCT02745119 (2016) phase 3 |
| Complement pathway inhibitor | CLG561 | Inhibits properdin | NCT01835015 (2013) phase 1; NCT02515942 (2015) phase 2 |
| Complement pathway inhibitor | APL-2 | Inhibits complement C3 | NCT000473928 (2017) phase 1; NCT02503332 (2018) phase 1; NCT03525613 (2018) phase 3; NCT03525600 (2018) phase 3; NCT04770545 (2021) phase 3 |
| Complement pathway inhibitor | LFG316; tesidolumab | Inhibits complement C5 | NCT01255462 (2010) phase 1; NCT02515942 (2015) phase 2; NCT01527500 (2012) phase 2 |
| Corticosteroid | Iluvien; fluocinolone acetonide | Inflammation suppression | NCT00695318 (2008) phase 2 |
| Gene therapy | AAVCAGsCD59; HMR59 | AAV2 gene therapy for transgene product CD59, inhibits membrane attack complex | NCT03144999 (2017) phase 1; NCT04358471 (2020) phase 2 |
| Neuropeptide | Brimonidine tartrate | α2 adrenergic agonist, prevents retinal ganglion cell death | NCT00658619 (2008) phase 1; NCT02087085 (2014) phase 2 |
| Neuropeptide | Ciliary neurotrophic factor | Prevent photoreceptor degradation | NCT00447954 (2007) phase 2 |
| Mitochondrial protection | Elamipretide | Mitochondrial protection | NCT03891875 (2019) phase 2; NCT02848313 (2016) phase 1 |
AMD, age-related macular degeneration.
Literature search
We used all available public domains to search for scientific literature published in English. The key words included: risuteganib, luminate, ALG-1001, integrin inhibitor. There was no time limitation for published reports.
What are integrins?
Integrins are heterodimeric receptors composed of 24 unique combinations of α and β subunits. There are 18 types of α subunits and 8 types of β subunits (Figure 1). The two subunits interact non-covalently and bind to a wide variety of extracellular matrix components and cell surface receptors. Integrins regulate a multiplicity of cellular roles such as shape, orientation, movement, adhesion, proliferation, invasion, apoptosis and survival (5). As a result of these many roles, integrins are involved in inflammatory, angiogenesis, and fibrosis cascades. Previously approved pharmacologic indications for integrin inhibitors use include Crohn’s disease, ulcerative colitis, and multiple sclerosis (6).
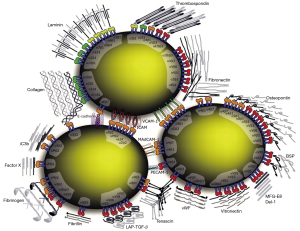
Lifitegrast was approved by the Food and Drug Administration in 2016 for the topical treatment of dry eye disease and is the first integrin inhibitor that has been successfully used in ophthalmology. It acts by inhibiting αLβ2 integrin which inhibits intercellular adhesion molecule 1 (ICAM-1) interaction, thereby preventing adhesion, activation, migration, and proliferation of lymphocytes. Left uninhibited, lymphocyte proliferation leads to cytokine secretion, cell destruction, and self-amplification of the inflammatory immune response that furthers the inflammatory mediators implicated in dry eye disease. Lifitegrast has been shown to be efficacious in the treatment of dry eye syndrome with no serious adverse effects (7-9).
Risuteganib
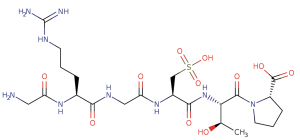
Risuteganib [Luminate (ALG-1001), Allegro Ophthalmics, CA, USA] is an intravitreally administered Arginine-Glycine-Aspartate (RGD) oligopeptide that has a molecular weight of less than 1 kDa (Figure 2). The RGD domain acts as a binding site for several extracellular matrix proteins such as fibronectin, fibrinogen, and vitronectin. Risuteganib acts by integrin inhibition, binding to 4 of the 24 known integrin heterodimers: αVβ3, αVβ5, α5β1, and αMβ2 (10,11). These integrins, specifically, are thought to be involved in pathways for angiogenesis, inflammation, and vascular permeability.
Takagi et al. showed increased expression of integrin αVβ3 and αVβ5 in murine models of ischemic retina. Through their association with vitronectin, these heterodimers are involved in angiogenesis and vascular proliferation (12). Similarly, Friedlander and colleagues found that enucleated eyes from patients with choroidal neovascularization (CNV) expressed αVβ3 while eyes from patients with retinal neovascularization expressed both αVβ3 and αVβ5 (13). These findings make these integrin heterodimers potentially useful therapeutic targets for angiogenic diseases such as AMD and diabetic macular edema (DME) (14-16). Friedlander and colleagues also demonstrated that murine retinal angiogenesis was dramatically reduced by systemic inhibition of αVβ3 and αVβ5 integrins (13).
Ramakrishnan et al. determined that integrin heterodimer α5β1 also plays a key role in angiogenesis as it is expressed in proliferating vascular endothelial cells. Inhibition of the α5β1-fibronectin interaction leads to apoptosis of proliferating endothelial cells but spares non-proliferating endothelial cells in vitro. Additionally, α5β1 integrin inhibition has an anti-angiogenic effect through a VEGF-independent pathway. In their study, Ramakrishnan et al. used a primate model of angiogenesis to determine that inhibition of α5β1 integrin may show promise in the treatment for ocular neovascularization (17).
Jawhara et al. used a murine model deficient in αMβ2 and found that the integrin heterodimer plays a role in the immune response through involvement in chemotaxis, inflammation, phagocytosis, and cell-mediated killing functions (18). Kim et al. determined that αMβ2 heterodimer is involved in monocyte adhesion through interaction with transforming growth factor-β-induced gene product (βig-h3/TGFBIp) (19). Transforming growth factor-β accumulation has been shown to be associated with diabetic angiopathy (20). Through intravitreal αMβ2 heterodimer inhibition, there is a potential to decrease inflammatory and pro-angiogenic chemokines.
As demonstrated by Yang et al., hydroquinone (an oxidant found in cigarette smoke and other pollutants) can induce necrosis and apoptosis, decrease mitochondrial bioenergetics, increase reactive oxygen species levels, and induce actin reorganization within human cultured RPE cells. They found that healthy RPE cells treated with risuteganib were not adversely affected, while RPE cells injured by hydroquinone but also treated with risuteganib were protected against injury (21). This suggests a potential role for risuteganib to treat diseases of RPE oxidative stress, such as AMD.
Pharmacokinetics and metabolism
Currently, there is no data available regarding the pharmacokinetics of risuteganib. Without this information, its duration of action or optimal timing of repeat doses remains unknown.
Preclinical studies
Preclinical trials have shown that risuteganib localizes to the RPE and outer retina and remains there for months. Radiolabeled risuteganib has been shown to have a 21-day half-life in rabbit retinal tissue. Retinas pretreated with risuteganib were protected from later exposure to the neurotoxic kainic acid or peroxide (22). Beltran et al. showed that RPE cells pretreated with risuteganib had a significant reduction in oxidative stress caused by hydrogen peroxide. Cells that were not pre-treated did not have the same cryoprotective effects (23).
Phase 1 studies
In a phase 1b study, 25 participants with exudative AMD received three monthly injections of either 1.5, 2.5, or 4.0 mg risuteganib and were followed for 6 months. The primary objective of the study was to observe for toxicity and to determine the maximally tolerated dose. Currently, there are no published results from this trial (24).
In a phase 1b/2a study, patients with DME received a monthly injection of either 1.5, 2.5, 5.0, or 7.0 mg risuteganib for 3 months and were followed for a total of 180 days. The primary endpoint of the study was to observe for toxicity and determine the maximally tolerated dose. Peak reduction in central macular thickness (CMT) by optical coherence tomography (OCT) ranged from 20% to 80% with a mean reduction of 31%. The treatment effect held for 3 additional months after the completion of treatment. Of the 15 patients enrolled, 53% had best corrected visual acuity (BCVA) improvement with a mean gain of nine or more letters (25,26).
Phase 2 studies
Forty patients were enrolled in a double masked, randomized, placebo controlled, multicenter trial evaluating the efficacy and safety of risuteganib treatment for intermediate-stage dry AMD. To be included in the study, patients needed to have non-exudative AMD with a BCVA between 20/40 and 20/100 (EDTRS between 33 and 73 letters). There was no mention of geographic atrophy in the inclusion or exclusion criteria. Patients were randomized to 1 of 2 study arms: two intravitreal injections of 1.0 mg risuteganib versus one sham injection in a 1.7:1 ratio. The risuteganib group received injections at week 1 and week 16 of the study. The study’s primary endpoint was to determine the proportion of patients in the risuteganib group with ≥8 ETDRS BCVA improvement at week 28 compared to the control group at week 12. The study found that more patients in the treatment group had a visual acuity improvement of eight or more letters (48%) compared to the control group (7.1%) (P=0.013) (27,28). There was no explanation as to the mechanism of vision gain in the treatment group.
A randomized, prospective, triple-masked phase 2 trial compared the safety and efficacy of risuteganib to bevacizumab in treating DME. For stage 1 of the trial, 138 participants were randomized to risuteganib monotherapy with 1.0, 2.0, or 3.0 or 1.25 mg bevacizumab. Injections were delivered monthly at weeks 0, 4, and 8 with an as needed injection at week 20 for the risuteganib groups and as needed injection at weeks 12, 16, or 20 for the bevacizumab groups. Patients who did not receive an as needed treatment at weeks 12, 16, or 20 were given a sham injection. A sham laser treatment was also administered at baseline and at week 16 for the patients receiving risuteganib. The risuteganib groups were compared to the bevacizumab groups at week 24. The primary endpoint was change in BCVA at week 24 compared to baseline. Risuteganib was found to be non-inferior to bevacizumab for the BCVA and CMT endpoints.
For stage 2 of the trial, 80 participants were randomized to 1 of 5 treatment groups. Group one was a control group and received monthly injections of 1.25 mg of bevacizumab for 5 months. Groups 2 and 3 received a single treatment of 1.25 mg bevacizumab at week 0 followed by three 1.0 mg (group 2) or 0.5 mg (group 3) risuteganib injections, at weeks 1, 4, and 8. Groups 4 and 5 received a combination of 1.0 or 0.5 mg of risuteganib, respectively, combined with bevacizumab 1.25 mg at weeks 1, 4, and 8. The most effective treatment was group 2 (1.25 mg bevacizumab at week 0 and three doses of 1.0 mg of risuteganib at weeks 1, 4, and 8), which met the primary endpoint of noninferiority in BCVA gain when compared to bevacizumab. There was a mean gain in BCVA of 7.1 letters for patients in the risuteganib with bevacizumab pre-treatment group (groups 2 and 3) compared to 6.7 letters for patients in the bevacizumab control group. Additionally, sequential risuteganib injections (groups 2 and 3) demonstrated 12-week durability. Sixty percent of patients treated in the trial had been chronic anti-VEGF users, which suggests that risuteganib may successfully treat patients who don’t respond to anti-VEGF. Inclusion criteria included BCVA of 20/50 to 20/320 (ETDRS equivalent 65 letters to 23 letters), treatment naïve, and clinically significant DME with central subfield thickness ≥350 µm by spectral domain OCT. Patients were excluded from the trial if they had active proliferative diabetic retinopathy, uncontrolled hypertension, or screening HbA1c blood test greater than 10.0% (29-31).
Phase 3 studies
Allegro Ophthalmics was preparing to enter the drug into phase 3 trials for AMD and DME starting in Q4 of 2020. As of the writing of this paper, no further information on timeline or study design was available (32).
Comparison studies
Schneider and colleagues created cybrid cells, which are cell lines created by fusing human RPE cells that lack mitochondrial DNA with platelets isolated from exudative and non-exudative AMD patients or age-matched normal subjects. A major factor in the development of AMD is mitochondrial damage and dysfunction. By isolating the mitochondria in the cybrid cells, the investigators were able to determine the consequences of patient-derived AMD mitochondria on RPE cells. They found that AMD cybrid cells treated with risuteganib had lower gene expression of the apoptotic regulator BAX, angiogenesis marker VEGF-A, and integrin ITGB1 genes than cells treated with bevacizumab and untreated control cells (33). This study demonstrated that monoclonal antibodies like bevacizumab may affect AMD RPE differently than risuteganib. Since risuteganib displayed more anti-apoptotic properties, it may be a promising therapy.
Safety and tolerability
There were no serious adverse events (SAE) attributed to the administration of intravitreal risuteganib in the trials for nonexudative AMD (25,30). The phase 2 trial for DME reported a total of 36 SAEs in the groups treated with at least one injection of risuteganib. The report detailed 6 cardiac disorders, 8 eye disorders, 2 general disorders, 6 infections, 2 metabolism and nutritional disorders, 1 musculoskeletal and connective tissue disorder, 1 neoplasm, 4 nervous system disorders, 1 renal and urinary disorder, 3 respiratory, thoracic, and mediastinal disorders, 1 surgical and medical procedure, and 1 vascular disorder (29). At this time, the detailed SAE data is not available for the nonexudative AMD trial. Longer term data regarding adverse events are currently not available and will be important to keep note of as risuteganib continues down the pipeline.
Conclusions
Integrins are heterodimeric receptors that play a role in the regulation of cellular adhesion, migration, proliferation, invasion, survival, and apoptosis. They have been shown to play a role in pathogenesis of DME and AMD. Integrin inhibition has been shown to be efficacious in the treatment of inflammatory diseases and provides an approach to impede the pathologic pathways of angiogenesis, inflammation, and vascular permeability in AMD and DME (6). Preclinical and early clinical studies show that risuteganib targets a novel integrin mediated pathway implicated in angiogenesis and inflammation. While intravitreal anti-VEGF treatments have had a dramatic impact on patient outcomes and quality of life, there is still an unmet clinical need for the treatment of non-exudative AMD.
An expert review of risuteganib by Shaw et al. concluded that while the current clinical results of risuteganib appear encouraging, clinicians should be skeptical about whether the clinical improvement in BCVA was truly an effect of risuteganib, especially given the short timeline of the studies and lack of pre-clinical data to explain the improvement in BCVA (34). A review by Samanta et al. also brings up concern with the crossing over of the phase 2 clinical trial occurring before the primary endpoint (35). Since these review, new non-clinical results remain promising, but there has been no further clinical data regarding risuteganib (21,33).
It is exciting that risuteganib improved BCVA in patients with dry AMD, but longer and larger trials will be needed to determine its true efficacy and mechanism of action. To date, studies assessing the actions of αVβ3 and αVβ5 integrins and preclinical trials, do not seem to offer an explanation for why risuteganib reverses retinal damage caused in non-exudative AMD, especially in such a short period of time. However, Elamipretide, another drug undergoing clinical trials for the treatment of dry AMD, also demonstrated an increase in BVCA for patients with dry and exudative AMD after a short study period (28 weeks) (36). With its novel mechanism of action, risuteganib has potential in the treatment of non-exudative AMD as monotherapy, and in DME and exudative AMD as adjunctive therapy to anti-VEGF agents or secondary therapy in refractory cases.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sayena Jabbehdari) for the series “Novel Treatment in Age-related Macular Degeneration” published in Annals of Eye Science. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/aes-21-12
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aes-21-12). The series “Novel Treatment in Age-related Macular Degeneration” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106-16. [Crossref] [PubMed]
- Hanus J, Zhao F, Wang S. Current therapeutic developments in atrophic age-related macular degeneration. Br J Ophthalmol 2016;100:122-7. [Crossref] [PubMed]
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials 1999;20:573-600. [Crossref] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005-15. [Crossref] [PubMed]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992;69:11-25. [Crossref] [PubMed]
- Ley K, Rivera-Nieves J, Sandborn WJ, et al. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov 2016;15:173-83. [Crossref] [PubMed]
- Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology 2014;121:475-83. [Crossref] [PubMed]
- Tauber J, Karpecki P, Latkany R, et al. Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study. Ophthalmology 2015;122:2423-31. [Crossref] [PubMed]
- Holland EJ, Luchs J, Karpecki PM, et al. Lifitegrast for the Treatment of Dry Eye Disease: Results of a Phase III, Randomized, Double-Masked, Placebo-Controlled Trial (OPUS-3). Ophthalmology 2017;124:53-60. [Crossref] [PubMed]
- Quiroz-Mercado H. Integrin Peptide Therapy in Choroidal and Retinal Neovascularization. Retina Today. 2013;30-1.
- Risuteganib (LUMINATE®): potential Paradigm Shift In the Treatment of Oxidative Stress-Induced DME. Allegro Ophthalmics, LLC, 2018 [Last accessed 2021 March 2]. Available online: https://ois.net/wp-content/uploads/2018/10/CompanyShowcase_Allegro.pdf
- Takagi H, Suzuma K, Otani A, et al. Role of vitronectin receptor-type integrins and osteopontin in ischemia-induced retinal neovascularization. Jpn J Ophthalmol 2002;46:270-8. [Crossref] [PubMed]
- Friedlander M, Theesfeld CL, Sugita M, et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A 1996;93:9764-9. [Crossref] [PubMed]
- Das UN. Diabetic macular edema, retinopathy and age-related macular degeneration as inflammatory conditions. Arch Med Sci 2016;12:1142-57. [Crossref] [PubMed]
- Telander DG. Inflammation and age-related macular degeneration (AMD). Semin Ophthalmol 2011;26:192-7. [Crossref] [PubMed]
- Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000;45:115-34. [Crossref] [PubMed]
- Ramakrishnan V, Bhaskar V, Law DA, et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol 2006;5:273-86. [PubMed]
- Jawhara S, Pluskota E, Cao W, et al. Distinct Effects of Integrins αXβ2 and αMβ2 on Leukocyte Subpopulations during Inflammation and Antimicrobial Responses. Infect Immun 2016;85:e00644-16. [PubMed]
- Kim HJ, Kim IS. Transforming growth factor-beta-induced gene product, as a novel ligand of integrin alphaMbeta2, promotes monocytes adhesion, migration and chemotaxis. Int J Biochem Cell Biol 2008;40:991-1004. [Crossref] [PubMed]
- Adki KM, Kulkarni YA. Potential Biomarkers in Diabetic Retinopathy. Curr Diabetes Rev 2020;16:971-83. [Crossref] [PubMed]
- Yang P, Shao Z, Besley NA, et al. Risuteganib Protects against Hydroquinone-induced Injury in Human RPE Cells. Invest Ophthalmol Vis Sci 2020;61:35. [Crossref] [PubMed]
- The Scoop on Anti-Integrin Therapy for DME. PU Dugel. Retina Today, 2019 [Last accessed 2021 March 2]. Available online: http://retinatoday.com/2019/02/the-scoop-on-anti-integrin-therapy-for-dme
- Beltran MA, Zamora-Alvarado R, Gonzalez-Salinas R, et al. Cytoprotective effect of ALG-1001 peptide (Luminate) on human retinal pigment epithelial cells exposed to oxidative injury. A novel functional-outcome for an anti-VEGF agent. Invest Ophthalmol Vis Sci 2018;59:1465.
- A Safety and Efficacy Study of ALG-1001 In Human Subjects With Wet Age-Related Macular Degeneration. NIH/U.S. National Library of Medicine. ClinicalTrials.gov, 2021 [Last accessed 2021 March 2]. Available online: https://clinicaltrials.gov/ct2/show/NCT01749891
- The Promise of Integrin Receptors for Treating Vitreoretinal Disorders. V Gonzalez. Retina Today, 2016 [Last accessed 2021 March 2]. Available online: https://retinatoday.com/articles/2016-july-aug/the-promise-of-integrin-receptors-for-treating-vitreoretinal-disorders
- Safety Study of ALG 1001 to Treat Diabetic Macular Edema. NIH/U.S. National Library of Medicine. ClinicalTrials.gov, 2021 [Last accessed 2021 March 2]. Available online: https://clinicaltrials.gov/ct2/show/NCT01482871
- Allegro Ophthalmics Announces Positive Topline Vision Results of Phase 2 Study Evaluating Risuteganib in Patients with Intermediate Dry Age-Related Macular Degeneration. Allegro Ophthalmics, LLC, 2019 [Last accessed 2021 March 2]. Available online: https://www.allegroeye.com/allegro-ophthalmics-expands-its-anti-integrin-portfolio-with-new-front-of-the-eye-drug-candidate-alg-1007-for-the-treatment-of-dry-eye-disease-3-2-2/
- A Randomized Controlled, Double-Masked, Crossover Clinical Trial Designed to Evaluate the Safety And Exploratory Efficacy Of 1.0 Mg Luminate® (Alg-1001) as A Treatment for Non-Exudative Macular Degeneration. NIH/U.S. National Library of Medicine. ClinicalTrials.gov, 2021 [Last accessed 2021 March 2]. Available online: https://clinicaltrials.gov/ct2/show/NCT03626636
- A Phase 2 Randomized, Controlled, Double-Masked, Multicenter Clinical Trial Designed to Evaluate the Safety and Exploratory Efficacy of Luminate® (ALG-1001) as Compared to Avastin® in the Treatment of Diabetic Macular Edema (DME). NIH/U.S. National Library of Medicine. ClinicalTrials.gov, 2021 [Last accessed 2021 March 2]. Available online: https://clinicaltrials.gov/ct2/show/NCT02348918
- Allegro Ophthalmics Announces Positive Topline Results from DEL MAR Phase 2b Stage 2 Trial Evaluating Luminate® in Patients with Diabetic Macular Edema. Allegro Ophthalmics, LLC. 2017 [Last accessed 2021 March 2]. Available online: https://www.allegroeye.com/allegro-ophthalmics-announces-positive-topline-results-from-del-mar-phase-2b-stage-2-trial-evaluating-luminate-in-patients-with-diabetic-macular-edema-2/
- Quiroz-Mercado H, Boyer DS, Campochiaro PA, et al. Randomized, Prospective, Double-Masked, Controlled Phase 2b Trial to Evaluate the Safety & Efficacy of ALG-1001 (Luminate®) in Diabetic Macular Edema. Invest Ophthalmol Vis Sci 2018;59:1960.
- Pipeline. Allegro Ophthalmics, LLC. 2020 [Last accessed 2021 March 2]. Available online: https://www.allegroeye.com/pipeline
- Schneider K, Chwa M, Atilano SR, et al. Differential effects of risuteganib and bevacizumab on AMD cybrid cells. Exp Eye Res 2021;203:108287. [Crossref] [PubMed]
- Shaw LT, Mackin A, Shah R, et al. Risuteganib-a novel integrin inhibitor for the treatment of non-exudative (dry) age-related macular degeneration and diabetic macular edema. Expert Opin Investig Drugs 2020;29:547-54. [Crossref] [PubMed]
- Samanta A, Aziz AA, Jhingan M, et al. Emerging Therapies in Nonexudative Age-Related Macular Degeneration in 2020. Asia Pac J Ophthalmol (Phila) 2021;10:408-16. [Crossref] [PubMed]
- McCarthy R. Stealth Biothereupetics: Elamipretide. 2019 [Last accessed 2021 March 2]. Available online: https://www.stealthbt.com/wp-content/uploads/Stealth-Biotherapeutics-Innovation-Showcase-at-Ophthalmology-Innovation-Summit-@-ASRS-2019.mp4
Cite this article as: Solinski MA, Raiji VR. Narrative review of risuteganib for the treatment of dry age-related macular degeneration (AMD). Ann Eye Sci 2021;6:36.


