
Narrative review-drug delivery in age-related macular degeneration
Introduction
Age-related macular degeneration (AMD), a progressive disease affecting the central retina, is the leading cause of irreversible blindness in industrialized countries and accounts for 8.7% of blindness worldwide (1). Age is the leading risk factor for development of AMD, affecting 4.8% of people ages 65 and older, and up to 25% of people by age 80 (2,3). In the early stages of AMD (also known as “dry” AMD), the clinical presentation can range from asymptomatic to blurred central vision that impairs daily activities. It is pathologically characterized by thickening of Bruch’s membrane due to yellow, subretinal deposits of lipid-rich proteins called drusen (4,5). The buildup of drusen disrupts both fluid efflux across Bruch’s membrane and cholesterol metabolism, causing oxidative stress on the RPE and accumulation of lipid peroxidation byproducts (6,7). Dry AMD can remain stable and minimally symptomatic for many years. However, 20.2% of cases progress to advanced disease which contributes to 90% of AMD-related blindness (8-10).
Advanced AMD can be classified into two subtypes: late-stage dry AMD [known as geographic atrophy (GA)], and neovascular (“wet”) AMD (nAMD). GA results from progressive, irreversible loss of photoreceptor and retinal pigment epithelium (RPE) cells by the mechanism described above (11). Wet AMD is believed to arise from abnormal blood vessel growth from the choroid into the normally avascular subretinal and sub-RPE layers, a process known as choroidal neovascularization (CNV) (3,5). CNV is thought to be a multifactorial consequence of drusen accumulation, disrupted choroidal blood supply to the RPE, and hypoxic conditions that induce the expression of angiogenic signaling proteins (5). If untreated, nAMD can lead to retinal exudation, sub-macular hemorrhage, and subretinal fibrosis that significantly impairs vision.
The treatment of nAMD has significantly advanced since the era of thermal laser photocoagulation and photodynamic therapy (PDT) two to three decades ago (12). Currently, intravitreal anti-vascular endothelial growth factor (VEGF) injection is the gold-standard therapy that can maintain or improve visual acuity in the majority of patients with nAMD. However, to date, no treatment exists for progression of dry AMD or GA (13).
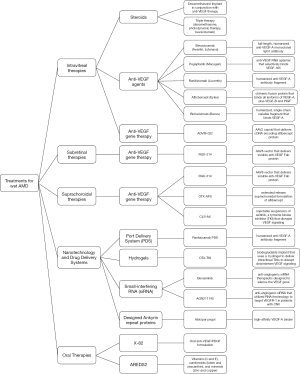
This literature review aims to highlight standard-of-care and clinical trial evaluation of drug delivery routes for the treatment of wet AMD, and therapies currently being evaluated for both neovascular and non-neovascular AMD (Figures 1,2). Numerous drug targets and therapeutic routes are currently being explored and exciting advances are currently under clinical investigation (14-17). The goal of our review is to summarize therapies that are currently in clinical use, therapies under investigation in clinical trials, and therapeutics that did not meet their clinical trial endpoint.
The review was conducted using Pubmed to identify peer-reviewed journal articles using the keywords age-related macular degeneration, anti-vascular endothelial growth factor, ocular gene therapy, and other related search terms between the years of 1990 to 2020. ClinicalTrials.gov was used to gather data on current clinical trials not yet published in the literature. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aes-21-8).
Intravitreal therapies
Intravitreal injection (IVI) of anti-VEGF agents is currently the standard of care for nAMD (18). This delivery route has become well-established in vitreoretinal practices and is safe and efficacious. IVI is a simple, minimally-invasive delivery method that is used to provide efficient, in-office treatment. Topical anesthesia is sufficient for the majority of patients, though subconjunctival anesthetic can also be administered. A 30-gauge needle (or smaller) is typically used to administer 0.05cc of drug, although up to 0.1cc of fluid can be safely tolerated during IVI with minimal transient rise in intraocular pressure (19,20).
Despite their efficacy, IVIs still carry a risk for visually-significant complications that increases with the number of injections (21). Risks include endophthalmitis, intraocular inflammation, rhegmatogenous retinal detachment (RRD), and IOP elevation (22-24). Not only do these complications increase with the number of injections, but the four to eight week anti-VEGF injection schedule typically required to control exudation poses a significant treatment burden on the patient, provider, and entire healthcare system. Consequently, the frequency of injections administered in clinical trials is often greater than the frequency in real-world clinical practice. Additionally, the course of disease is variable between patients, and those with greater neovascular activity or bilateral disease may require more frequent visits which may lead to treatment fatigue. These burdens, in part, account for the discrepancy in visual outcomes of nAMD patients between clinical trials versus observational studies, with the former showing a 1 to 2 letter-line improvement (25-29) and the latter only approximately 1 letter-line improvement (30-34).
Steroid therapies
Intravitreal corticosteroids are commonly used as a standalone therapy for various retinal diseases, including diabetic macular edema (DME), cystoid macular edema (CME), uveitis, and retinal vascular occlusion (RVO) (35). Steroids induce the production of various proteins that inhibit the release of arachidonic acid, a critical player in the expression of pro-inflammatory markers, from cellular plasma membranes (35,36). Among many cellular growth factors that have been isolated in ocular neovascularization, VEGF has been shown to play a key role in the pathogenesis of nAMD (8,37). Steroids lead to downstream inhibition of VEGF expression and decreased blood vessel permeability, and were thus thought to have therapeutic potential for treatment of nAMD. However, studies have failed to show steroid monotherapy to be beneficial, and it is therefore, not recommended (36,38).
A few small randomized trials have evaluated adjunctive steroid administration in eyes resistant to anti-VEGF monotherapy. Despite anatomical improvements in retinal fluid absorption, no benefit for visual acuity was seen in patients receiving a dexamethasone implant in conjunction with anti-VEGF therapy (aflibercept or ranibizumab) compared to controls receiving anti-VEGF therapy alone (39). However, older literature has shown that triple therapy consisting of PDT, dexamethasone, and bevacizumab can improve visual acuity, stabilize vision, and potentially reduce the number of anti-VEGF injections required (40-42). Statistical significance failed to be proven due to small sample sizes, and given the development of new therapies and efficacy of current anti-VEGF monotherapy, triple therapy is uncommon for the treatment of nAMD in clinical settings today.
Anti-VEGF intravitreal therapies
The first systemically administered anti-VEGF agent available on the market was bevacizumab (Avastin®), a full-length, humanized anti-VEGF-A monoclonal IgG1 antibody that was FDA approved in 2004 for the treatment of metastatic colon cancer (43). Later that year, pegaptanib (Macugen®), an anti-VEGF RNA aptamer that selectively binds VEGF-165 with high affinity, was the first ocular anti-VEGF therapy FDA approved for nAMD treatment (44). Pegaptanib decreased the rate of visual-acuity loss, progression to blindness, and the number of letters lost by 50% after 1 year of treatment (45). Shortly thereafter, an open-label, uncontrolled clinical study found that systemic infusion of bevacizumab was also efficacious for nAMD treatment (46). Clinicians began using off-label bevacizumab IVIs which were later shown to improve functional visual outcomes in nAMD patients (47). The use of pegaptanib was consequently discontinued by many clinicians due to the availability of more effective treatments.
The off-label use of bevacizumab was efficacious, but researchers believed a treatment with a smaller molecular weight than bevacizumab [149 kilodaltons (kDa)] could potentially reach the choroid more efficiently. Ranibizumab (Lucentis®, 48 kDa), a humanized anti-VEGF-A antibody fragment that utilizes an affinity-matured Fab region derived from the same parent molecule as bevacizumab (48), was subsequently developed specifically for intravitreal nAMD treatment and FDA approved in 2006. Ranibizumab was the first ocular anti-VEGF drug to significantly improve visual acuity and prevent vision loss in nAMD patients after one year of monthly treatment (25).
In 2011, The Comparison of Age-Related Macular Degeneration Treatment Trials (CATT) found that intravitreal bevacizumab and ranibizumab treatment had equivalent effects on functional visual outcomes (49). Minor differences in visual acuity improvement were seen between patients receiving as-needed treatments of either drug compared to those receiving monthly treatments after both 1 and 2 years (27). That same year, the FDA approved another intravitreal nAMD treatment: aflibercept (Eylea®), a chimeric fusion protein consisting of the second immunoglobulin (Ig) domain of VEGF receptor (R)-1, third Ig binding domain of VEGFR-2, and Fc region of human IgG1 (50). Aflibercept uniquely binds all isoforms of VEGF-A, in addition to VEGF-B, and placental growth factor (PIGF) which is selectively expressed in pathological angiogenic tissue (51).
While ranibizumab and aflibercept have been FDA approved for wet AMD, off-label bevacizumab remains a common treatment choice due to its significantly lower cost and non-inferior clinical efficacy (bevacizumab is 40 times cheaper than ranibizumab and aflibercept) (52). When comparing ranibizumab and aflibercept in treatment-naïve nAMD patients, no significant differences were found in visual acuity after 1 year of IVI (53). Similar anatomic response rates for macular thickness and subretinal fluid have been found between bevacizumab, ranibizumab, and aflibercept (54). Taken together, the choice of anti-VEGF therapy can usually be decided on an individual basis between providers and patients, with particular consideration regarding out-of-pocket-cost and treatment administration frequency.
An ophthalmic formulation of bevacizumab, ONS-5010 (Lytenava®), is currently being investigated in the NORSE 2 Phase III clinical trial (55). The NORSE 1 Phase II trial showed no statistical differences in eyes treated with intravitreal ONS-5010 compared to eyes treated with intravitreal ranibizumab in safety measures, visual acuity, and letter-line improvement after 11 months of treatment. Results from the Phase III trial are expected in 2021, and if successful, ONS-5010 will be the first FDA-approved formulation of bevacizumab to be used for retinal indications.
Brolucizimab is the most recent anti-VEGF therapy to be FDA approved in 2019. With a molecular weight of only 26 kDa, this humanized, single-chain variable fragment binds VEGF-A with greater bioavailability than previous therapies (56). Visual acuity at 2 years was shown to be non-inferior when administered at every 3 month intervals as compared to bimonthly aflibercept injections (57). However, reports of occlusive vasculitis after brolicuzimab injection have led to apprehension in the ophthalmology community in adopting its use (58).
Anti-VEGF intravitreal gene therapy
Gene therapy has recently emerged as a viable treatment method for monogenic hereditable dystrophies and is undergoing investigation for retinal degenerative diseases such as AMD. In the latter case, the goal of intravitreal gene therapy is to offer a long-lasting, single-dose treatment that has sustained therapeutic effect through creating a protein biofactory (20). The eye is a particularly good candidate for gene therapy for several reasons: (I) it is easily accessible, (II) treatment can be monitored using non-invasive technology, such as optical coherence tomography (OCT), (III) ocular immune privilege reduces the risk of significant host-immune response, (IV) an effective therapeutic dose may be achieved with a smaller quantity of viral vector, and (V) systemic exposure is limited due to anatomical barriers inherent to the eye (59,60). Luxturna® (voretigine neparvovec-rzyl) was the first ocular gene therapy to be FDA approved in 2017 for treatment of Leber congenital amaurosis type 2 (LCA2) (61), pioneering the era of gene therapy for inherited and acquired ocular diseases alike. Luxturna utilizes an adeno-associated virus (AAV)-based viral vector, a delivery vehicle shared by many other ocular gene therapies (62,63), to deliver a functional copy of the RPE65 gene (64). Several anti-VEGF gene therapy treatments are also being investigated for both dry AMD and wet AMD. Table 1 summarizes the different ocular routes of administration for gene and non-gene therapies for AMD.
Table 1
| Route of administration | Advantages | Disadvantages |
|---|---|---|
| Intravitreal | Minimally invasive; commonly used; efficient, in-office procedure | Increased humoral response to intravitreal gene therapy; requires higher dose to achieve therapeutic effect at the outer retina or RPE |
| Subretinal | Direct drug delivery to RPE, which is particularly useful in gene therapy; greater immune privilege when compared to intravitreal injection | Requires PPV and creation of a retinotomy; potentially challenging surgical approach that is not yet commonly used; longer procedure time; procedure associated complications including cataract formation and retinal detachment |
| Suprachoroidal | Does not require creation of a retinotomy; high bioavailability in the RPE, sclera, and choroid; avoids PPV-associated complications | Novel surgical approach that is not yet commonly used; longer procedure time; theoretical increased risk of suprachoroidal hemorrhage |
| Topical (eye drops) | Convenient and accessible; non-invasive; do not require patient to come to physician’s office | Poor bioavailability in the posterior segment; increased systemic exposure to the medication |
| Systemic (oral) | ||
| Systemic (intravenous) | Minimal risk of ocular complications |
IOP, intraocular pressure; RPE, retinal pigment epithelium; RRD, rhegmatogenous retinal detachment.
ADVM-022 (Adverum Biotechnologies) is a novel, adeno-associated virus (AAV)-based therapy that utilizes an AAV2 capsid to deliver cDNA encoding the aflibercept protein (65). This therapy is currently being investigated in the ongoing phase I OPTIC trial (66). As of June 2020, 2 cohorts of 6 eyes each have been enrolled in the study and have received one IVI of ADVM-022 at 6×1011 vg/eye and 2×1011 vg/eye, respectively. Complications were limited to inflammation related to the injection. No subject has required rescue anti-VEGF injection since initial ADVM-022 treatment, with visual acuity being maintained and imaging showing improvements in retinal anatomy across all eyes. The efficacy of this treatment is promising, and further results are underway. However, several challenges inherent to the intravitreal delivery route exist. Intravitreal viral vectors are limited in their ability to transduce RPE or photoreceptor cells, the most common retinal gene therapy targets, due to the physical barriers imposed by the internal limiting membrane and retinal layers (67). To overcome these physical limitations of IVIs, subretinal delivery of gene therapy has also been evaluated as a delivery route for ocular gene therapy.
Subretinal (SR) therapies
The SR space offers direct access to the diseased RPE, but subretinal delivery is significantly more difficult and invasive when compared to IVIs (68). A protocol recently published by Davis et al. (69) details the procedure for SR delivery of gene therapy as follows: (I) surgical preparation, including vector preparation and injection site planning; (II) pars plana vitrectomy and induction of a posterior vitreous detachment; (III) creation of a retinotomy, and optional creation of a pre-bleb with balanced salt solution, forming a localized retinal detachment between the RPE and photoreceptor layer; and (IV) vector injection via a subretinal microcannula into the bleb. A limitation of this technique is that only relatively small areas of the retina can be treated at a time. Thus, multiple retinotomies may be required to treat the desired retinal surface area. The procedure poses the typical risks of vitreoretinal surgery, including cataract development, retinal detachment, and possible vision loss (69). Additionally, vector material may reflux into the vitreous through the injection site and lead to formation of an epiretinal membrane that can worsen visual outcomes (70). Despite the challenges associated with SR drug delivery, it is particularly important in certain inherited retinal disorders to target the SR space when administering gene or cell therapy. This method of drug delivery continues to be explored as an important modality for gene therapies.
Subretinal anti-VEGF gene therapy
A current Phase I/IIa trial using RGX-314 (Regenxbio) has shown promising results utilizing a novel AAV-8 vector to subretinally deliver a soluble anti-VEGF Fab protein (71). Five different doses of RGX-314 (3×109, 1×1010, 6×1010, 1.6×1011, and 2.5×1011 genome copies/eye) were evaluated for safety and efficacy in 42 eyes previously treated with nAMD therapy. As of August 2020, no immune or inflammatory responses beyond what is normally expected post-retinal surgery were observed, and all confirmed adverse effects during the trial have not been drug-related. Cohorts 3 (6×1010 genome copies/eye), 4 (1.6×1011 genome copies/eye), and 5 (2.5×1011 genome copies/eye) have shown the best results in the study thus far, with a 62.2%, 61.3%, and 84.5% decrease seen in annual anti-VEGF injection rates respectively.
Suprachoroidal approach
The suprachoroidal space (SCS) is another attractive space for drug delivery applied in AMD and other retinal degenerations. It has been evaluated as an effective site for drug delivery using various molecular and protein-based therapies (72-77). Due to its close proximity to the retina and RPE, the SCS also offers the potential for low drug doses with high bioavailability in the posterior segment (78). Drug delivery into the SCS also avoids the risks associated with retinal surgeries while potentially offering similar immune privilege to SR drug delivery.
Recent clinical trials have shown successful distribution of ocular therapies to the SR space via SCS delivery (79). Three different SCS delivery methods have been described in the literature thus far. Freehand injection using a standard hypodermic needle is the simplest approach for drug delivery to the SCS: the surgeon advances the needle through the sclera and into the SCS at a tangential angle, and injects the drug which diffuses within the SCS (80). This method has been evaluated in preclinical models but the difficulty in reproducible SCS access precludes clinical use. Alternatively, a much shorter, 700-µm microneedle has been tested for transscleral injection in in-vivo animal models (81) and involves a protocol similar to IVI. This technique is planned to be incorporated in clinical trials evaluating suprachoroidal anti-VEGF gene therapy for nAMD. Lastly, a flexible, tunneled microcatheter has been used to allow suprachoroidal and suprachoroidal-to-subretinal delivery to the posterior pole. The surgeon creates a limbal conjunctival peritomy followed by a radial scleral cut down, allowing the catheter to enter the SCS and be guided to its target site using indirect ophthalmoscopy (82). Preclinical studies have shown an acceptable safety profile for these SCS approaches without severe adverse events such as suprachoroidal hemorrhage (82-86). Clinical trials are also planned using tunneled microcatheters for suprachoroidal-to-subretinal gene therapy in AMD with GA.
Suprachoroidal anti-VEGF therapy
Preclinical trials have evaluated gene therapy delivery into the SCS using animal models and have suggested diffuse distribution in the RPE 2 weeks following injection (87). A single injection of RGX-314, the same AAV8 vector expressing anti-VEGF that was described in a previous section, successfully suppressed CNV in rodent eyes within 2 weeks of administration, and maintained similar findings on evaluation 7 weeks after treatment (80). Additionally, no differences in anti-VEGF levels in the retina were found between therapy delivered in the SR space versus the SCS.
In September 2020, Ocular Therapeutix® announced the development of OTX-AFS, an extended-release suprachoroidal formulation of aflibercept, following promising, preliminary results in their phase I trial on OTX-TKI (described in the section on hydrogels) (88). A protocol for a phase I trial is scheduled to be released soon.
Additionally, the FDA recently approved initiation of a phase I/IIa clinical trial evaluating suprachoroidal delivery of CLS-AX (Clearside Biomedical), an injectable suspension containing axitinib (89). Axitinib is a tyrosine kinase inhibitor (TKI) that disrupts downstream VEGF signaling, and is currently approved for treatment of renal cell cancer. Preclinical data in animal models has shown CLS-AX to be durable in the SCS, well-tolerated, and successful in preventing further CNV (90). This trial is set to begin enrolling within the coming months.
Nanotechnology and other drug delivery systems
Advancements in nanotechnology and biomaterials have led to the development of microscopic drug delivery systems (DDSs), including hydrogels, microparticles, nanoparticles, implants, and liposomes (91,92). The use of DDSs for ocular therapy offers many advantages that make them an attractive alternative to the current mainstay treatment of IVIs. These include improved retinal penetration and tapered release of highly concentrated drug therapies (93), which both serve to reduce the treatment and surveillance burden. However, loading DDSs with anti-VEGF molecules and other protein-based therapies is more complicated from a pharmacological standpoint due to their fragile tertiary and quaternary structures (94). The technique involved in delivering various DDSs will be discussed in each subsection when applicable.
Port delivery systems (PDS)
PDS has shown promise for long-lasting delivery of anti-VEGF medication that may decrease IVI treatment burden for nAMD. The PDS implant is a novel drug delivery system that acts as a depot and continuously delivers a therapeutic agent (i.e., ranibizumab) into the vitreous cavity over extended periods of time (95). Prior to surgical insertion, local anesthesia is administered (96). Scleral implantation is accomplished via conjunctival peritomy, scleral dissection using a 3.2 mm blade, 532 nm laser ablation of the exposed pars plana, and insertion of the self-retaining device into the scleral opening (96). Once implanted, the drug passively diffuses from the release control element into the vitreous, offering sustained-release until emptied. Similar to IVIs, the PDS can be refilled in-office using a small needle to inject new fluid into the implant.
Recent results from the LADDER phase II trial of a ranibizumab PDS show similar functional and anatomical visual outcomes as monthly intravitreal ranibizumab (95). No significant differences were found between time to first refill across the 40-mg/mL, and 100-mg/mL PDS dosage-arms, with median refill times ranging from 13 to 15 months. The 10-mg/mL arm had a median refill time of 8.7 months, which is still a significantly lower treatment burden than monthly IVIs (95). As expected, higher rates of adverse effects were noted in the PDS arm when compared to IVI, including vitreous hemorrhage, nausea, and headache. However, these events were not severe and did not cause significant visual acuity deficits (97). Endophthalmitis was also a complication of the technique.
The phase III ARCHWAY study is currently underway and is assessing safety and non-inferiority of 24-week PDS dosing to monthly IVI delivery of ranibizumab. Initial results have successfully proven non-inferiority, with a 0.5 letter gain in the monthly injection arm to a 0.2 letter gain in the PDS arm, and only 2% of subjects requiring a medication refill in the PDS arm prior to the scheduled 24-week refill (98).
Hydrogels
To overcome the surgical risks associated with PDS, injectable hydrogels are being explored as potential extended-release DDSs for ocular drug therapy (14). Hydrogels are water-soluble DDSs that form three-dimensional, cross-linked polymer networks (99). They offer varying levels of porosity, distensibility, and mechanical resistance, and these features can be modulated through natural, synthetic, or hybrid polymers (14). When incorporating therapeutic agents into the polymer network, each gel’s unique cross-linking properties must be accounted for to ensure bioactivity upon arrival to the target site (100-103).
To date, only one ocular hydrogel DDS has reached clinical trials. OTX-TKI (Ocular Therapeutix) is an injectable, biodegradable implant that utilizes a hydrogel to deliver intravitreal TKIs to disrupt downstream VEGF signaling (104,105). Once implanted into the vitreous, OTX-TKI offers sustained drug-release for up to 6–7 months (105). Pre-clinical in vitro studies in Dutch belted rabbits demonstrated successful delivery and maintenance of high OVX-TKI concentrations in the vitreous using this therapy (106). Precise information regarding the structural design of OTX-TKI is currently unavailable, though a presentation regarding this novel technology given by Ocular Therapeutix in October 2020 advertises a polyethylene glycol hydrogel delivery platform (107). The current phase I trial has fully enrolled two cohorts using 200 µg, and 400 µg doses, respectively, and a third cohort is in the process of being enrolled to compare 600 to 400 µg + anti-VEGF induction injection. According to the most recent presentation, subjects in the first two cohorts have demonstrated a favorable safety profile. In cohort 2 (400 µg), one patient demonstrated treatment durability of 9 months, and a few subjects showed decreased retinal fluid on OCT by 2 months. Comprehensive results are still underway, and will be closely followed.
Polymers
The brimonidine DDS (Allergan) is a biodegradable intravitreal implant made from a polymer matrix (14). It is loaded with brimonidine tartrate, a selective alpha-2 adrenergic receptor agonist, and is currently used to treat glaucoma. Brimonidine has been shown to reduce oxidative stress in the RPE (108) and has a neuroprotective effect in in vitro models of retinal degeneration (109). Results from the recent phase II clinical trial showed the brimonidine DDS to be a safe and effective treatment of GA secondary to dry AMD (110). When compared to sham groups, eyes treated with 132 and 264 µg of brimonidine via DDS had significantly less growth in geographic atrophy lesion size. A phase III trial is currently in the process of being developed.
Small-interfering RNA (siRNA)
siRNA is a class of double-stranded RNA molecules that regulates gene expression via RNA interference (RNAi) (111), a biological process that inhibits gene expression via degradation of complementary messenger RNA (112). siRNA-based therapies have recently emerged as promising treatments for nAMD, offering translational modulation of pathological protein production, convenient intravitreal delivery, the potential to elicit a pan-retinal effect, and a high level of specificity (113,114). A few ocular drugs in this class have been developed to target the main pathway involved in the pathogenesis of AMD: the pro-angiogenic VEGF pathway (115).
Bevasirinib, an anti-angiogenic siRNA therapeutic designed to silence the VEGF gene using an RNAi mechanism, was the first intravitreal siRNA therapy developed for nAMD (115,116). Despite being successful in both in vitro and in vivo models (117), the phase III trial was discontinued in 2009 due to failure to show bevasirinib injections at 8 to 12 week intervals (after initial pre-treatment with 3 ranibizumab injections) to be more effective than monthly ranibizumab injections (118,119). AGN211745 (also known as siRNA-027) is another anti-angiogenic siRNA that utilized RNAi technology to target VEGFR-1 in patients with CNV (120). Similar to bevasirinib, development of AGN211745 was halted in 2009 following a phase II trial due to insignificant improvements in visual acuity (121).
Recent advances in siRNA therapy include the FDA approval of ONPATTRO® in 2018 and GIVLAARITM in 2019 for use in hereditary transthyretin-mediated amyloidosis and acute hepatic porphyria, respectively (122). RNA-based drug therapies utilizing RNAi mechanisms are thus effective treatments, and further research is warranted for future AMD therapies of this nature.
Designed ankyrin repeat proteins
Ankyrin repeats are naturally occurring amino acid motifs that perform various cellular functions, including cell-cell signaling, cell cycle regulation, and transcription (123). These repeats serve as high-affinity scaffolding domains for high-specificity interactions with target receptors (124,125) and are great candidates for drug delivery due to their stable thermodynamic properties (126). Designed ankyrin repeat domains (DARPins) were engineered to mimic these protein structures. This platform was first introduced in ocular therapeutics as abicipar pegol, a high-affinity VEGF-A binder (127) delivered via IVI with up to 5.5 times more concentrated dosages than ranibizumab (128).
The recent phase II REACH trial compared functional and anatomical visual outcomes in naive nAMD patients randomized into three arms: 0.5 mg IVI of ranibizumab, 1 mg IVI of abicipar, or 2 mg IVI of abicipar (128). Results were promising and showed similar best corrected visual acuity and reduction in central retinal thickness across all arms. This prompted the phase III SEQUOIA and CEDAR clinical trials to assess the efficacy and safety of abicipar. Non-inferiority was established when comparing abicipar to ranibizumab, and treatment burden was shown to be lower with 8-week and 12-week abicipar regimens (129). However, intraocular inflammation (IOI) rates were relatively high after administration of abicipar, affecting 10.4% of patients in phase II trials and 15.3% of patients in phase III trials (130). Although reported IOI rates for current intravitreal anti-VEGF range from 0.3–14.3% (131-135), the FDA did not approve abicipar for clinical use in nAMD patients due to the unfavorable risk-benefit ratio. Despite the shortcomings of abicipar’s safety profile, DARPins are promising, advanced DDSs that should continue to be explored as potential AMD treatments.
Oral therapy
Oral therapy is perhaps the most common and convenient route for systemic drug administration. Oral drug dosages can be easily adjusted, self-administered, is non-invasive, and has the potential for solid formulations to have a long shelf-life (136). For patients with systemic diseases and concomitant ocular manifestations, oral administration is effective and reduces patients’ total drug intake (137). However, for patients with localized eye disease, oral dosages are not as appealing due to the many ocular barriers preventing efficient drug delivery to the posterior segment. Higher dosages or greater frequency of oral drug administration may thus be required to achieve the desired therapeutic effect, posing a significant risk of drug toxicity (138). For this reason, X-82, an oral anti-VEGF/PDGF formulation designed for nAMD treatment, failed to demonstrate safety and tolerability in phase II trials despite promising results in previous trials (139). Oral drug administration has also been shown to produce an ocular bioavailability of only 2% (140), making this route of drug delivery inefficient for AMD and other localized ocular diseases.
Age-Related Eye Disease Studies (AREDS) 1 and 2 were large, multicenter phase III trials that analyzed the effects of nutritional supplements and dietary modifications on AMD progression (141,142). Oral supplementation of specific combinations of vitamins (C and E), carotenoids (lutein and zeaxanthin), and minerals (zinc and copper) was shown to decrease the risk of progression to late stages of AMD and GA (143,144). Adherence to a Mediterranean dietary pattern with high intake of omega-3 fatty acids decreased risk of progression to GA as well (145). Due to minimal risks associated with these supplements and dietary changes and the potential for disease-sparing benefits, some ophthalmologists recommend these to patients diagnosed with early stages of AMD.
Several other oral pharmaceuticals are being investigated in clinical trials for their potential therapeutic effects in patients with GA. Oral doxycycline (Oracea®) is currently being investigated in a phase III trial for its anti-inflammatory effects and potential to reduce the rate of GA spread (146). Metformin, a commonly prescribed drug for type II diabetes mellitus, has been noted for its role in preventing AMD development (147,148). It activates retinal 5' adenosine monophosphate-activated protein kinase (AMPK) and glucose metabolism, and these two mechanisms have been shown to be protective of the RPE and photoreceptor layers in rodent models (149). An ongoing phase II trial is evaluating the safety and efficacy of metformin for progression of GA in non-diabetic patients, and results are expected in October 2021 (150).
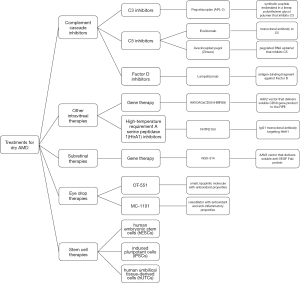
Dry AMD therapies
To date, there are no FDA approved therapies for dry AMD progression or GA despite being common precursors to vision loss. The total area of GA grows geometrically over time, and mean time to central involvement is 2.5 years. Bilateral eye involvement, on average, occurs 7 years after initial GA diagnosis (151,152). GA development is not exclusive to dry AMD, as studies have shown up to 17–18% of nAMD patients receiving anti-VEGF treatment develop GA within 2 years (153,154). These numbers translate to devastating effects on patients’ vision and quality of life, and the prevalence of advanced AMD is projected to double by 2040 (1). Thus, treatments for prevention of GA progression are becoming more crucial as the global population continue to age. The complement cascade has been implicated as a central factor in the multifactorial pathogenesis of GA (11), and therapeutics that target the inhibition of various complement factors are currently in trials.
Complement cascade inhibitors
C5 is an effector molecule produced by all three pathways of the complement cascade and plays a key role in forming the membrane attack complex (MAC) (155). Chronic activation of MAC may lead to death of the RPE, and therapies that selectively inhibit C5 may mitigate this pathogenic complement dysregulation seen in advanced forms of AMD (155,156). In 2007, eculizumab, a monoclonal human antibody to C5, was first FDA approved for treatment of paroxysmal nocturnal hemoglobinuria. By blocking C5, eculizumab prevents downstream activation of the terminal complement system and inhibits formation of the MAC. It was thus thought to be a potential systemic treatment for GA, and was investigated in the COMPLETE phase II study. Thirty patients were randomized into one of three intravenous treatment groups: low dose eculizumab (600 mg for 4 weeks, then 900 mg every other week for 20 weeks), high dose eculizumab (900 mg for 4 weeks, then 1,200 mg every other week for 20 weeks), or placebo (157). At both 26 weeks and 52 weeks of follow-up, eculizumab failed to decrease GA lesion progression when compared to placebo (P=0.96 and P=0.93, respectively). However, no subjects progressed to wet AMD, and thus a different study endpoint with longer follow-up may be warranted to determine its efficacy in this regard.
Avacincaptad pegol (Zimura®), a pegylated RNA aptamer delivered via IVI, is a novel C5-inhibitor currently in phase III trials for treatment of GA (158). A total of 286 subjects with GA secondary to AMD were randomized into one of three avacincaptad dosage groups during the first part of the phase II trial: 1 mg, 2 mg, and sham (159). The second part of this trial randomized 209 subjects into 2 mg avacincaptad, 4 mg avacincaptad, and sham cohorts. The results from this trial showed that avacincaptad reduced the rate of atrophic area spread by 27.4% and 27.8% in the 2 and 4 mg treatment groups respectively (159), had a 0% incidence rate of endophthalmitis, and a favorable safety profile with no drug-related adverse events. Ongoing phase III trials are aiming to confirm the efficacy of avacincaptad, and if successful, will be the first treatment option available to individuals with GA.
C3 is an upstream component of C5 in the complement cascade, and marks the convergence of the three complement pathways that may ultimately lead to cell lysis and inflammation (160). C3 plays a key role in complement cascade amplification, particularly through the alternative pathway (161,162), and its breakdown products are markedly elevated in serum plasma of patients with GA (163). Pegcetacoplan (APL-2) was the first complement inhibitor to enter clinical trials for treatment of dry AMD and GA. It is a synthetic peptide embedded in a linear, polyethylene glycol polymer, and its potent inhibition of C3 offers the potential for broad anti-inflammatory effects (164). Phase II trials showed a 29% reduction in the GA growth rate during a 12-month period in patients receiving monthly intravitreal pegcetacoplan, and a 20% reduction in patients receiving treatment every other month when compared to sham injections (165). 1.2% of patients enrolled in the trial developed endophthalmitis. The phase III trial is currently underway and has enrolled 1, 259 patients. The primary aims are to further evaluate APL-2’s efficacy in reducing rates of GA spread and its safety profile. Results of this phase III trial are expected in 2021.
Factor D is an important activator of C3 convertase, and acts as the rate-limiting enzyme of the alternative pathway (166,167). It was thus thought to be a viable therapeutic target for treatment of dry AMD, leading to the development of lampalizumab, an antigen-binding fragment against Factor D. In a phase II trial, lampalizumab IVI successfully reduced GA lesion growth by 20% in an 18-month period when compared to sham (168). However, Chroma and Spectri, two identical phase III trials, failed to show significant differences in slowing GA progression over 48 weeks with every 4 or 6 week lampalizumab injection. These trials found lesions to enlarge by an average of 2 mm2 each year, consistent with the natural rate of GA progression described in the literature (13), and were thus suspended in September 2017 (169).
Other intravitreal therapies
Gene therapy is also being explored to target components of the complement pathway. CD59 is a natural inhibitor of MAC (170), and is currently being studied as a therapeutic target for both dry and wet AMD. AAVCAGsCD59 (HMR59) is an IVI that uses an AAV2 vector to deliver soluble CD59 gene product to the RPE (171). Phase I trials evaluating HMR59 are currently underway for both forms of AMD. Another gene therapy being investigated for dry AMD is subretinal GT005, which uses an AAV2 vector to code for complement factor I (CFI) (172). CFI is a serum protease that can inhibit the entire complement cascade (173), and pre-clinical trials in rodent models have shown dose-dependent decreases in complement activation and CNV when treated with GT005 (172). There have yet to be any safety concerns in the ongoing phase I/II trials in the United Kingdom, and results are forthcoming.
High-temperature requirement A serine peptidase 1 (HtrA1) has been implicated in the pathogenesis of geographic atrophy, inducing breakdown of the extracellular matrix protein and degeneration of photoreceptors and RPE. Locus variations of the ARMS2/HTRA1 gene have been implicated to increase risk of dry AMD development and progression (174). FHTR2163 is an IgG1 monoclonal antibody targeting HtrA1 administered as intravitreal injections every 4 to 8 weeks. The phase I open-label trial met safety criteria and a phase II clinical trial (GALLEGO) is currently enrolling patients to determine its efficacy in reducing GA lesion progression and safety profile (175).
Eye drop therapies
Eye drop therapies are commonly used for treatment of anterior segment pathologies. Topical drug delivery to the posterior segment is limited due to inherent ocular barriers, including its many structural layers, dynamic blood flow, and presence of efflux pumps (140). Despite this challenge, eye drop formulations continue to be explored due to their convenience and ease of use. OT-551 is a small, lipophilic molecule with antioxidant properties (176). Due to its ability to penetrate membranes and protective properties of antioxidants, OT-511 was investigated in a phase II trial in 2010 for its efficacy in reducing GA progression (176,177). Ten patients were treated with topical 0.45% OT-551 monocularly for 2 years in a randomized fashion; the fellow eye served as a control. Though the primary outcome of reducing the GA lesion progression failed to be met, a decrease in visual acuity was significantly lower in the treatment eye than in the fellow eye (176). However, larger studies failed to replicate this effect and OT-551 did not progress to larger clinical trials. MC-1101, a vasodilator with antioxidant and anti-inflammatory properties, successfully demonstrated safety and tolerability in a phase Ib trial (178). Phase II/III trials are currently underway to investigate its efficacy in maintaining visual function (179).
Stem cell therapies
Stem cells are promising vehicles for tissue regeneration and if successful, may revolutionize the prognosis of degenerative diseases. Research on ocular stem cell therapy has involved cells from various cell lines in both pre-clinical and clinical trials, including human embryonic stem cells (hESCs) (180-182), induced pluripotent cells (iPSCs) (183), and human umbilical tissue-derived cells (hUTCs) (79). The intended effects of ocular cell therapy depend on the site of delivery and type of cell. In some cases, the goal is for cells to secrete a trophic, immunomodulatory or neuroprotective factor while in others the cells directly replace damaged or degenerated RPE or photoreceptors (184).
A phase I trial involving 2 patients successfully delivered an RPE patch derived from hESCs, with significant visual improvements of 21 and 29 letter-line after 12 months (180). Another phase I/IIa trial enrolled 16 subjects with advanced atrophic AMD, and demonstrated the feasibility of subretinal implantation of an hESC-derived RPE monolayer (181). The National Eye Institute is currently launching a phase I/IIa trial of 12 subjects with GA to evaluate the safety of transplanting an RPE monolayer from autologous iPSCs (183).
In human clinical trials, the use of hESC and iPSC-derived therapies is still in its early stages, with under 100 patients undergoing transplantation thus far (185). Limitations of stem cell therapy include the risk of oncogenicity, need for more efficient cell-line generation, and lack of knowledge regarding the translation of results seen in animal studies to human subjects. The journey ahead for stem cell therapy is exciting, but many challenges must be overcome before it enters clinical use.
Conclusions
The introduction of anti-VEGF therapies as the gold standard for nAMD has been associated with a 46–50% decrease in vision loss attributable to nAMD (186,187). However, standard-of-care monthly intravitreal dosing is a burdensome treatment regimen for many patients. Gene therapy and stem cell therapy have shown promising early results in clinical trials, as have various PDSs and nanotechnology therapies. They offer the potential for an expanded range of therapeutic opportunities for patients with nAMD and/or GA. Aside from oral vitamin/mineral supplementation, no current treatments exist to ameliorate progression to advanced AMD. Significant unresolved gaps in knowledge exist in both disease-modifying drug targets and drug/delivery systems which are an important area of study. A critical need for treatment of dry AMD and GA remains unmet and is a driving force for the development of new drug therapies and delivery options in AMD research.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sayena Jabbehdari) for the series “Novel Treatment in Age-related Macular Degeneration” published in Annals of Eye Science. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/aes-21-8
Peer Review File: Available at https://dx.doi.org/10.21037/aes-21-8
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aes-21-8). The series “Novel Treatment in Age-related Macular Degeneration” was commissioned by the editorial office without any funding or sponsorship. Dr. DX received consulting fees from Gyroscope Therapeutics and Alimera Sciences. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106-16. [Crossref] [PubMed]
- Owen CG, Jarrar Z, Wormald R, et al. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 2012;96:752-6. [Crossref] [PubMed]
- Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron 2012;75:26-39. [Crossref] [PubMed]
- De Jong PT. Elusive drusen and changing terminology of AMD. Eye (Lond) 2018;32:904-14. [Crossref] [PubMed]
- Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond) 2016;3:34. [Crossref] [PubMed]
- Bowes Rickman C, Farsiu S, Toth CA, et al. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci 2013;54:ORSF68-80. [Crossref] [PubMed]
- Ebrahimi KB, Handa JT. Lipids, Lipoproteins, and Age-Related Macular Degeneration. Journal of Lipids 2011;2011:802059. [Crossref] [PubMed]
- Gehrs KM, Anderson DH, Johnson LV, et al. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med 2006;38:450-71. [Crossref] [PubMed]
- Seddon JM, Chen CA. The Epidemiology of Age-Related Macular Degeneration. Int Ophthalmol Clin 2004;44:17-39. [Crossref] [PubMed]
- Flaxel CJ, Adelman RA, Bailey ST, et al. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology 2020;127:1-65. [Crossref] [PubMed]
- Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, et al. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina (Philadelphia, Pa) 2017;37:819-35. [Crossref] [PubMed]
- Bakri SJ, Thorne JE, Ho AC, et al. Safety and Efficacy of Anti-Vascular Endothelial Growth Factor Therapies for Neovascular Age-Related Macular Degeneration: A Report by the American Academy of Ophthalmology. Ophthalmology 2019;126:55-63. [Crossref] [PubMed]
- Fleckenstein M, Mitchell P, Freund KB, et al. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018;125:369-90. [Crossref] [PubMed]
- Jemni-Damer N, Guedan-Duran A, Fuentes-Andion M, et al. Biotechnology and Biomaterial-Based Therapeutic Strategies for Age-Related Macular Degeneration. Part I: Biomaterials-Based Drug Delivery Devices. Front Bioeng Biotechnol 2020;8:549089. [Crossref] [PubMed]
- Jemni-Damer N, Guedan-Duran A, Fuentes-Andion M, et al. Biotechnology and Biomaterial-Based Therapeutic Strategies for Age-Related Macular Degeneration. Part II: Cell and Tissue Engineering Therapies. Front Bioeng Biotechnol 2020;8:588014. [Crossref] [PubMed]
- Hadziahmetovic M, Malek G. Age-Related Macular Degeneration Revisited: From Pathology and Cellular Stress to Potential Therapies. Front Cell Developmental Biol 1872;2021:8.
- Scheive M, Yazdani S, Hajrasouliha AR. The utility and risks of therapeutic nanotechnology in the retina. Ther Adv Ophthalmol 2021;13:25158414211003381. [Crossref] [PubMed]
- Lanzetta P, Loewenstein A. Vision Academy Steering C. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol 2017;255:1259-73. [Crossref] [PubMed]
- Kampougeris G, Spyropoulos D, Mitropoulou A. Intraocular Pressure rise after Anti-VEGF Treatment: Prevalence, Possible Mechanisms and Correlations. J Curr Glaucoma Pract 2013;7:19-24. [Crossref] [PubMed]
- Xu D, Khan MA, Ho AC. Creating an Ocular Biofactory: Surgical Approaches in Gene Therapy for Acquired Retinal Diseases. Asia Pac J Ophthalmol (Phila) 2021;10:5-11. [Crossref] [PubMed]
- Jager RD, Aiello LP, Patel SC, et al. Risks of intravitreous injection: a comprehensive review. Retina 2004;24:676-98. [Crossref] [PubMed]
- Moshfeghi AA. Endophthalmitis following intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Semin Ophthalmol 2011;26:139-48. [Crossref] [PubMed]
- Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27:787-94. [Crossref] [PubMed]
- Hoang QV, Mendonca LS, Della Torre KE, et al. Effect on intraocular pressure in patients receiving unilateral intravitreal anti-vascular endothelial growth factor injections. Ophthalmology 2012;119:321-6. [Crossref] [PubMed]
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration. N Engl J Med 2006;355:1432-44. [Crossref] [PubMed]
- Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013;120:1046-56. [Crossref] [PubMed]
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-98. [Crossref] [PubMed]
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537-48. [Crossref] [PubMed]
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med 2006;355:1419-31. [Crossref] [PubMed]
- Cohen SY, Mimoun G, Oubraham H, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina 2013;33:474-81. [Crossref] [PubMed]
- Finger RP, Wiedemann P, Blumhagen F, et al. Treatment patterns, visual acuity and quality-of-life outcomes of the WAVE study - a noninterventional study of ranibizumab treatment for neovascular age-related macular degeneration in Germany. Acta Ophthalmol 2013;91:540-6. [Crossref] [PubMed]
- Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015;99:220-6. [Crossref] [PubMed]
- Rao P, Lum F, Wood K, et al. Real-World Vision in Age-Related Macular Degeneration Patients Treated with Single Anti–VEGF Drug Type for 1 Year in the IRIS Registry. Ophthalmology 2018;125:522-8. [Crossref] [PubMed]
- Ozturk M, Harris ML, Nguyen V, et al. Real-world visual outcomes in patients with neovascular age-related macular degeneration receiving aflibercept at fixed intervals as per UK licence. Clin Exp Ophthalmol 2018;46:407-11. [Crossref] [PubMed]
- Sarao V, Veritti D, Boscia F, et al. Intravitreal Steroids for the Treatment of Retinal Diseases. The Scientific World Journal 2014;2014:989501. [Crossref] [PubMed]
- Wang Y, Wang VM, Chan CC. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye (Lond) 2011;25:127-39. [Crossref] [PubMed]
- Lopez PF, Sippy BD, Lambert HM, et al. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1996;37:855-68. [PubMed]
- Chaudhary V, Barbosa J, Lam WC, et al. Ozurdex in age-related macular degeneration as adjunct to ranibizumab (The OARA Study). Can J Ophthalmol 2016;51:302-5. [Crossref] [PubMed]
- Giancipoli E, Pinna A, Boscia F, et al. Intravitreal Dexamethasone in Patients with Wet Age-Related Macular Degeneration Resistant to Anti-VEGF: A Prospective Pilot Study. J Ophthalmol 2018;2018:5612342. [Crossref] [PubMed]
- Ehmann D, García R. Triple therapy for neovascular age-related macular degeneration (verteporfin photodynamic therapy, intravitreal dexamethasone, and intravitreal bevacizumab). Can J Ophthalmol 2010;45:36-40. [Crossref] [PubMed]
- Augustin AJ, Puls S, Offermann I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: verteporfin PDT, bevacizumab, and dexamethasone. Retina 2007;27:133-40. [Crossref] [PubMed]
- Bakri SJ, Couch SM, McCannel CA, et al. Same-day triple therapy with photodynamic therapy, intravitreal dexamethasone, and bevacizumab in wet age-related macular degeneration. Retina 2009;29:573-8. [Crossref] [PubMed]
- Kazazi-Hyseni F, Beijnen JH, Schellens JHM. Bevacizumab. Oncologist 2010;15:819-25. [Crossref] [PubMed]
- Kim LA, D'Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol 2012;181:376-9. [Crossref] [PubMed]
- Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for Neovascular Age-Related Macular Degeneration. N Engl J Med 2004;351:2805-16. [Crossref] [PubMed]
- Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 2005;112:1035-47. [Crossref] [PubMed]
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2005;36:331-5. [Crossref] [PubMed]
- Blick SK, Keating GM, Wagstaff AJ. Ranibizumab. Drugs 2007;67:1199-206; discussion 1207-9. [Crossref] [PubMed]
- CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-908. [Crossref] [PubMed]
- Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859-70. [Crossref] [PubMed]
- Van Bergen T, Etienne I, Cunningham F, et al. The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases. Prog Retin Eye Res 2019;69:116-36. [Crossref] [PubMed]
- van Asten F, Michels CTJ, Hoyng CB, et al. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration-A cost-effectiveness analysis from a societal perspective. PLoS One 2018;13:e0197670. [Crossref] [PubMed]
- Böhni SC, Bittner M, Howell JP, et al. Comparison of Eylea® with Lucentis® as first-line therapy in patients with treatment-naïve neovascular age-related macular degeneration in real-life clinical practice: retrospective case-series analysis. BMC Ophthalmol 2015;15:109. [Crossref] [PubMed]
- Park DH, Sun HJ, Lee SJ. A comparison of responses to intravitreal bevacizumab, ranibizumab, or aflibercept injections for neovascular age-related macular degeneration. Int Ophthalmol 2017;37:1205-14. [Crossref] [PubMed]
- Outlook Therapeutics Reports Topline Results and Positive Proof-of-Concept for ONS-5010 / LYTENAVA™ (bevacizumab-vikg) from NORSE 1 Outlook Therapeutics: Outlook Therapeutics; 2020 Available online: http://www.globenewswire.com/news-release/2020/08/26/2084007/0/en/Outlook-Therapeutics-Reports-Topline-Results-and-Positive-Proof-of-Concept-for-ONS-5010-LYTENAVA-bevacizumab-vikg-from-NORSE-1.html
- Nguyen QD, Das A, Do DV, et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020;127:963-76. [Crossref] [PubMed]
- Sharma A, Kumar N, Bandello F, et al. Brolucizumab: the road ahead. Br J Ophthalmol 2020;104:1631-2. [Crossref] [PubMed]
- Rosenfeld PJ, Browning DJ. Is This a 737 Max Moment for Brolucizumab? Am J Ophthalmol 2020;216:A7-8. [Crossref] [PubMed]
- Peng Y, Tang L, Zhou Y. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res 2017;58:217-26. [Crossref] [PubMed]
- Li S, Datta S, Brabbit E, et al. Nr2e3 is a genetic modifier that rescues retinal degeneration and promotes homeostasis in multiple models of retinitis pigmentosa. Gene Ther 2021;28:223-241. [Crossref] [PubMed]
- Fuller-Carter PI, Basiri H, Harvey AR, et al. Focused Update on AAV-Based Gene Therapy Clinical Trials for Inherited Retinal Degeneration. BioDrugs 2020;34:763-81. [Crossref] [PubMed]
- Rodrigues GA, Shalaev E, Karami TK, et al. Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye. Pharm Res 2018;36:29. [Crossref] [PubMed]
- Ramlogan-Steel CA, Murali A, Andrzejewski S, et al. Gene therapy and the adeno-associated virus in the treatment of genetic and acquired ophthalmic diseases in humans: Trials, future directions and safety considerations. Clin Exp Ophthalmol 2019;47:521-36. [Crossref] [PubMed]
- Maguire AM, Bennett J, Aleman EM, et al. Clinical Perspective: Treating RPE65-Associated Retinal Dystrophy. Mol Ther 2021;29:442-63. [Crossref] [PubMed]
- Grishanin R, Vuillemenot B, Sharma P, et al. Preclinical Evaluation of ADVM-022, a Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol Ther 2019;27:118-29. [Crossref] [PubMed]
- Khanani AM, Kiss S, Turpcu A, et al. Phase 1 Study of Intravitreal Gene Therapy ADVM-022 for neovascular AMD (OPTIC Trial). Invest Ophthalmol Visual Sci 2020;61:1154.
- Naylor A, Hopkins A, Hudson N, et al. Tight Junctions of the Outer Blood Retina Barrier. Int J Mol Sci 2019;21:211. [Crossref] [PubMed]
- Davis JL. The Blunt End: Surgical Challenges of Gene Therapy for Inherited Retinal Diseases. Am J Ophthalmol 2018;196:xxv-xxix. [Crossref] [PubMed]
- Davis JL, Gregori NZ, MacLaren RE, et al. Surgical Technique for Subretinal Gene Therapy in Humans with Inherited Retinal Degeneration. Retina 2019;39:S2-8. [Crossref] [PubMed]
- Spencer R, Fisher S, Lewis GP, et al. Epiretinal membrane in a subject after transvitreal delivery of palucorcel (CNTO 2476). Clin Ophthalmol 2017;11:1797-803. [Crossref] [PubMed]
- Wykoff CC, editor Gene Therapy with RGX-314 for Neovascular AMD: New Results from the Ongoing Phase I/IIa Study. Retina Society 2020 VR; 2020 August.
- Patel SR, Berezovsky DE, McCarey BE, et al. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci 2012;53:4433-41. [Crossref] [PubMed]
- Wang M, Liu W, Lu Q, et al. Pharmacokinetic comparison of ketorolac after intracameral, intravitreal, and suprachoroidal administration in rabbits. Retina 2012;32:2158-64. [Crossref] [PubMed]
- Kim YC, Edelhauser HF, Prausnitz MR. Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles. Invest Ophthalmol Vis Sci 2014;55:7387-97. [Crossref] [PubMed]
- Patel SR, Lin AS, Edelhauser HF, et al. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res 2011;28:166-76. [Crossref] [PubMed]
- Hackett SF, Fu J, Kim YC, et al. Sustained delivery of acriflavine from the suprachoroidal space provides long term suppression of choroidal neovascularization. Biomaterials 2020;243:119935. [Crossref] [PubMed]
- Yeh S, Khurana RN, Shah M, et al. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology 2020;127:948-55. [Crossref] [PubMed]
- Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev 2018;126:58-66. [Crossref] [PubMed]
- Heier JS, Ho AC, Samuel MA, et al. Safety and Efficacy of Subretinally Administered Palucorcel for Geographic Atrophy of Age-Related Macular Degeneration: Phase 2b Study. Ophthalmol Retina 2020;4:384-93. [Crossref] [PubMed]
- Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest 2019;129:4901-11. [Crossref] [PubMed]
- Yiu G, Chung SH, Mollhoff IN, et al. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol Ther Methods Clin Dev 2020;16:179-91. [Crossref] [PubMed]
- Olsen TW, Feng X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol 2006;142:777-87. [Crossref] [PubMed]
- Einmahl S, Savoldelli M, D'Hermies F, et al. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest Ophthalmol Vis Sci 2002;43:1533-9. [PubMed]
- Chen M, Li X, Liu J, et al. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release 2015;203:109-17. [Crossref] [PubMed]
- Gu B, Liu J, Li X, et al. Real-Time Monitoring of Suprachoroidal Space (SCS) Following SCS Injection Using Ultra-High Resolution Optical Coherence Tomography in Guinea Pig Eyes. Invest Ophthalmol Vis Sci 2015;56:3623-34. [Crossref] [PubMed]
- Kim YC, Edelhauser HF, Prausnitz MR. Particle-stabilized emulsion droplets for gravity-mediated targeting in the posterior segment of the eye. Adv Healthc Mater 2014;3:1272-82. [Crossref] [PubMed]
- Habot-Wilner Z, Noronha G, Wykoff CC. Suprachoroidally injected pharmacological agents for the treatment of chorio-retinal diseases: a targeted approach. Acta Ophthalmol 2019;97:460-72. [Crossref] [PubMed]
- BioSpace. Ocular Therapeutix™ and AffaMed Therapeutics Announce License Agreement and Collaboration for DEXTENZA® and OTX-TIC in Asia 2020 Available online: https://www.biospace.com/article/releases/ocular-therapeutix-and-affamed-therapeutics-announce-license-agreement-and-collaboration-for-dextenza-and-otx-tic-in-asia/
- Biomedical C. Clearside Biomedical Announces U.S. FDA Acceptance of Investigational New Drug Application for CLS-AX (axitinib injectable suspension) Administered in Suprachoroidal Space 2020 Available online: https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedical-announces-us-fda-acceptance-investigational
- Kaiser PK, Ciulla T, Kansara V. Suprachoroidal CLS-AX (axitinib injectable suspension), as a Potential Long-Acting Therapy for Neovascular Age-Related Macular Degeneration (nAMD). Invest Ophthalmol Visual Sci 2020;61:3977.
- Kang-Mieler JJ, Rudeen KM, Liu W, et al. Advances in ocular drug delivery systems. Eye 2020;34:1371-9. [Crossref] [PubMed]
- Luaces-Rodríguez A, Mondelo-García C, Zarra-Ferro I, et al. Intravitreal anti-VEGF drug delivery systems for age-related macular degeneration. Int J Pharm 2020;573:118767. [Crossref] [PubMed]
- Zarbin MA, Montemagno C, Leary JF, et al. Nanotechnology in ophthalmology. Can J Ophthalmol 2010;45:457-76. [Crossref] [PubMed]
- Oo C, Kalbag SS. Leveraging the attributes of biologics and small molecules, and releasing the bottlenecks: a new wave of revolution in drug development. Taylor & Francis; 2016.
- Khanani AM, Callanan D, Dreyer R, et al. End of Study Results for the Ladder Phase 2 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmol Retina 2020;S2468-6530(20)30447-4.
- Campochiaro PA, Marcus DM, Awh CC, et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019;126:1141-54. [Crossref] [PubMed]
- Chen ER, Kaiser PK. Therapeutic Potential of the Ranibizumab Port Delivery System in the Treatment of AMD: Evidence to Date. Clin Ophthalmol 2020;14:1349-55. [Crossref] [PubMed]
- Charters L. ASRS 2020: PDS with ranibizumab signals paradigm shift in treatment of neovascular AMD: Ophthalmology Times; 2020 [July 27, 2020: Available online: https://www.modernretina.com/view/asrs-2020-pds-with-ranibizumab-signals-paradigm-shift-in-treatment-of-neovascular-amd
- Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater 2016;1:16071. [Crossref] [PubMed]
- Kang Derwent JJ, Mieler WF. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans Am Ophthalmol Soc 2008;106:206-13; discussion 213-4. [PubMed]
- Matanović MR, Kristl J, Grabnar PA. Thermoresponsive polymers: insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int J Pharm 2014;472:262-75. [Crossref] [PubMed]
- Awwad S, Al-Shohani A, Khaw PT, et al. Comparative Study of In Situ Loaded Antibody and PEG-Fab NIPAAM Gels. Macromol Biosci 2018;18: [Crossref] [PubMed]
- Awwad S, Abubakre A, Angkawinitwong U, et al. In situ antibody-loaded hydrogel for intravitreal delivery. Eur J Pharm Sci 2019;137:104993. [Crossref] [PubMed]
- Khan M, Agarwal K, Loutfi M, et al. Present and Possible Therapies for Age-Related Macular Degeneration. ISRN Ophthalmology 2014;2014:608390. [Crossref] [PubMed]
- Seah I, Zhao X, Lin Q, et al. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye (London, England) 2020;34:1341-56. [Crossref] [PubMed]
- Jarrett T, Elhayek RF, Lattrell Z, et al. Pharmacokinetics of a 6 month Sustained Hydrogel Delivery System for Tyrosine Kinase Inhibitors in Dutch Belted Rabbits. Invest Ophthalmol Visual Sci 2017;58:1984.
- Mattessich A. Transforming drug delivery leveraging a novel technology platform 2020 Available online: https://ocutx.gcs-web.com/static-files/0832ef68-8b94-4916-bce2-aacc61b0071e
- Tsao SW, Gabriel R, Thaker K, et al. Effects of Brimonidine on Retinal Pigment Epithelial Cells and Müller Cells Exposed to Amyloid-Beta 1-42 Peptide In Vitro. Ophthalmic Surg Lasers Imaging Retina 2018;49:S23-8. [Crossref] [PubMed]
- Rajagopalan L, Ghosn C, Tamhane M, et al. Cyto-/neuro-protective effects of brimonidine drug delivery system (DDS) in a nonhuman primate progressive retinal degeneration model of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). Invest Ophthalmol Visual Sci 2019;60:2993.
- Kuppermann BD, Patel SS, Boyer DS, et al. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (brimo dds) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina 2021;41:144-55. [Crossref] [PubMed]
- Dana H, Chalbatani GM, Mahmoodzadeh H, et al. Molecular Mechanisms and Biological Functions of siRNA. Int J Biomed Sci 2017;13:48-57. [PubMed]
- Lee YW, Hwang YE, Lee JY, et al. VEGF siRNA Delivery by a Cancer-Specific Cell-Penetrating Peptide. J Microbiol Biotechnol 2018;28:367-74. [Crossref] [PubMed]
- Gemayel MC, Bhatwadekar AD, Ciulla T. RNA therapeutics for retinal diseases. Expert Opin Biol Ther 2021;21:603-13. [Crossref] [PubMed]
- Pittella F, Miyata K, Maeda Y, et al. Pancreatic cancer therapy by systemic administration of VEGF siRNA contained in calcium phosphate/charge-conversional polymer hybrid nanoparticles. J Control Release 2012;161:868-74. [Crossref] [PubMed]
- Jiang J, Zhang X, Tang Y, et al. Progress on ocular siRNA gene-silencing therapy and drug delivery systems. Fundam Clin Pharmacol 2021;35:4-24. [Crossref] [PubMed]
- Singerman L. Combination therapy using the small interfering RNA bevasiranib. Retina 2009;29:S49-50. [Crossref] [PubMed]
- Xia XB, Xiong SQ, Song WT, et al. Inhibition of retinal neovascularization by siRNA targeting VEGF(165). Mol Vis 2008;14:1965-73. [PubMed]
- Garba AO, Mousa SA. Bevasiranib for the Treatment of Wet, Age-Related Macular Degeneration. Ophthalmology and Eye Diseases 2010;2:OED.S4878.
- Safety & Efficacy Study Evaluating the Combination of Bevasiranib & Lucentis Therapy in Wet AMD (COBALT) Available online: https://clinicaltrials.gov/ct2/show/NCT00499590
- Kaiser PK, Symons RC, Shah SM, et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol 2010;150:33-9.e2. [Crossref] [PubMed]
- Titze-de-Almeida R, David C, Titze-de-Almeida SS. The Race of 10 Synthetic RNAi-Based Drugs to the Pharmaceutical Market. Pharm Res 2017;34:1339-63. [Crossref] [PubMed]
- Hu B, Zhong L, Weng Y, et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther 2020;5:101. [Crossref] [PubMed]
- Mosavi LK, Cammett TJ, Desrosiers DC, et al. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci 2004;13:1435-48. [Crossref] [PubMed]
- Binz HK, Stumpp MT, Forrer P, et al. Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J Mol Biol 2003;332:489-503. [Crossref] [PubMed]
- Kohl A, Binz HK, Forrer P, et al. Designed to be stable: crystal structure of a consensus ankyrin repeat protein. Proc Natl Acad Sci U S A 2003;100:1700-5. [Crossref] [PubMed]
- Mosavi LK, Minor DL, Peng ZY. Consensus-derived structural determinants of the ankyrin repeat motif. Proc Natl Acad Sci U S A 2002;99:16029-34. [Crossref] [PubMed]
- Stahl A, Stumpp MT, Schlegel A, et al. Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis 2013;16:101-11. [Crossref] [PubMed]
- Callanan D, Kunimoto D, Maturi RK, et al. Double-Masked, Randomized, Phase 2 Evaluation of Abicipar Pegol (an Anti-VEGF DARPin Therapeutic) in Neovascular Age-Related Macular Degeneration. J Ocul Pharmacol Ther 2018;34:700-9. [Crossref] [PubMed]
- Partners M. Allergan and Molecular Partners Announce Two Positive Phase 3 Clinical Trials for Abicipar pegol 8 and 12-week Regimens for the Treatment in Patients with Neovascular Age-Related Macular Degeneration 2018 Available online: https://www.molecularpartners.com/allergan-and-molecular-partners-announce-two-positive-phase-3-clinical-trials-for-abicipar-pegol-8-and-12-week-regimens-for-the-treatment-in-patients-with-neovascular-age-related-macular-degeneration/
- Moisseiev E, Loewenstein A. Abicipar pegol—a novel anti-VEGF therapy with a long duration of action. Eye 2020;34:605-6. [Crossref] [PubMed]
- Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol 2011;56:95-113. [Crossref] [PubMed]
- Kitchens JW, Do DV, Boyer DS, et al. Comprehensive Review of Ocular and Systemic Safety Events with Intravitreal Aflibercept Injection in Randomized Controlled Trials. Ophthalmology 2016;123:1511-20. [Crossref] [PubMed]
- Khanani AM, Cohen GL, Zawadzki R. A Prospective Masked Clinical Assessment of Inflammation After Intravitreal Injection of Ranibizumab or Aflibercept. J Ocul Pharmacol Ther 2016;32:216-8. [Crossref] [PubMed]
- Souied EH, Dugel PU, Ferreira A, et al. Severe Ocular Inflammation Following Ranibizumab or Aflibercept Injections for Age-Related Macular Degeneration: A Retrospective Claims Database Analysis. Ophthalmic Epidemiol 2016;23:71-9. [Crossref] [PubMed]
- Goldberg RA, Shah CP, Wiegand TW, et al. Noninfectious inflammation after intravitreal injection of aflibercept: clinical characteristics and visual outcomes. Am J Ophthalmol 2014;158:733-7.e1. [Crossref] [PubMed]
- Homayun B, Lin X, Choi HJ. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019;11:129. [Crossref] [PubMed]
- Varela-Fernández R, Díaz-Tomé V, Luaces-Rodríguez A, et al. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020;12:269. [Crossref] [PubMed]
- Thrimawithana TR, Young S, Bunt CR, et al. Drug delivery to the posterior segment of the eye. Drug Discov Today 2011;16:270-7. [Crossref] [PubMed]
- Cohen MN, O'Shaughnessy D, Fisher K, et al. APEX: a phase II randomised clinical trial evaluating the safety and preliminary efficacy of oral X-82 to treat exudative age-related macular degeneration. Br J Ophthalmol 2021;105:716-22. [Crossref] [PubMed]
- Gaudana R, Ananthula HK, Parenky A, et al. Ocular drug delivery. Aaps j 2010;12:348-60. [Crossref] [PubMed]
- Group A-REDSR. The age-related eye disease study (AREDS): design implications AREDS report no 1. Controlled Clin Trials 1999;20:573. [Crossref] [PubMed]
- Chew EY, Clemons T, SanGiovanni JP, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012;119:2282-9. [Crossref] [PubMed]
- Chew EY, Clemons TE, Agrón E, et al. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 2013;120:1604-11.e4. [Crossref] [PubMed]
- Group A-REDSR. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417. [Crossref] [PubMed]
- Keenan TD, Agrón E, Mares J, et al. Adherence to the Mediterranean diet and progression to late age-related macular degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2020;127:1515-28. [Crossref] [PubMed]
- Clinical Study to Evaluate Treatment With ORACEA® for Geographic Atrophy (TOGA) Available online: https://clinicaltrials.gov/ct2/show/NCT01782989
- Brown EE, Ball JD, Chen Z, et al. The Common Antidiabetic Drug Metformin Reduces Odds of Developing Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2019;60:1470-7. [Crossref] [PubMed]
- Stewart JM, Lamy R, Wu F, et al. Relationship between Oral Metformin Use and Age-Related Macular Degeneration. Ophthalmol Retina 2020;4:1118-9. [Crossref] [PubMed]
- Xu L, Kong L, Wang J, et al. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc Natl Acad Sci U S A 2018;115:10475-80. [Crossref] [PubMed]
- Metformin for the Minimization of Geographic Atrophy Progression in Patients With AMD Available online: https://ClinicalTrials.gov/show/NCT02684578
- Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol 2009;127:1168-74. [Crossref] [PubMed]
- Feuer WJ, Yehoshua Z, Gregori G, et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of age-related eye disease study report no 26. JAMA Ophthalmol 2013;131:110-1. [Crossref] [PubMed]
- Thavikulwat AT, Jacobs-El N, Kim JS, et al. Evolution of Geographic Atrophy in Participants Treated with Ranibizumab for Neovascular Age-related Macular Degeneration. Ophthalmol Retina 2017;1:34-41. [Crossref] [PubMed]
- Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:150-61. [Crossref] [PubMed]
- Johnson LV, Leitner WP, Staples MK, et al. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res 2001;73:887-96. [Crossref] [PubMed]
- Wu J, Sun X. Complement system and age-related macular degeneration: drugs and challenges. Drug Des Devel Ther 2019;13:2413-25. [Crossref] [PubMed]
- Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology 2014;121:693-701. [Crossref] [PubMed]
- Csaky KG, Westby K, Rezaei K. Avacincaptad Pegol, A Novel C5 Inhibitor, Significantly Reduces the Mean Rate of Geographic Atrophy Growth in a Pivotal Clinical Trial. Invest Ophthalmol Visual Sci 2020;61:1943.
- Jaffe GJ, Westby K, Csaky KG, et al. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021;128:576-86. [Crossref] [PubMed]
- Ricklin D, Reis ES, Mastellos DC, et al. Complement component C3 - The "Swiss Army Knife" of innate immunity and host defense. Immunol Rev 2016;274:33-58. [Crossref] [PubMed]
- Harboe M, Garred P, Karlstrøm E, et al. The down-stream effects of mannan-induced lectin complement pathway activation depend quantitatively on alternative pathway amplification. Mol Immunol 2009;47:373-80. [Crossref] [PubMed]
- Harboe M, Ulvund G, Vien L, et al. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol 2004;138:439-46. [Crossref] [PubMed]
- Reynolds R, Hartnett ME, Atkinson JP, et al. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci 2009;50:5818-27. [Crossref] [PubMed]
- Apellis Completes Enrollment in Two Phase 3 Studies of the Targeted C3 Therapy, Pegcetacoplan, in Patients with Geographic Atrophy (GA) 2020 Available online: https://investors.apellis.com/news-releases/news-release-details/apellis-completes-enrollment-two-phase-3-studies-targeted-c3
- Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020;127:186-95. [Crossref] [PubMed]
- Park DH, Connor KM, Lambris JD. The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front Immunol 2019;10:1007. [Crossref] [PubMed]
- Lesavre PH, Müller-Eberhard HJ. Mechanism of action of factor D of the alternative complement pathway. J Exp Med 1978;148:1498-509. [Crossref] [PubMed]
- Yaspan BL, Williams DF, Holz FG, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med 2017;9:eaaf1443. [Crossref] [PubMed]
- Holz FG, Sadda SR, Busbee B, et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol 2018;136:666-77. [Crossref] [PubMed]
- Kumar-Singh R. The role of complement membrane attack complex in dry and wet AMD - From hypothesis to clinical trials. Exp Eye Res 2019;184:266-77. [Crossref] [PubMed]
- Bordet T, Behar-Cohen F. Ocular gene therapies in clinical practice: viral vectors and nonviral alternatives. Drug Discov Today 2019;24:1685-93. [Crossref] [PubMed]
- Ellis S, Buchberger A, Holder J, et al. GT005, a gene therapy for the treatment of dry age-related macular degeneration (AMD). Invest Ophthalmol Visual Sci 2020;61:2295.
- Alexander P, Gibson J, Cree AJ, et al. Complement factor I and age-related macular degeneration. Mol Vis 2014;20:1253-7. [PubMed]
- Tom I, Pham VC, Katschke KJ Jr, et al. Development of a therapeutic anti-HtrA1 antibody and the identification of DKK3 as a pharmacodynamic biomarker in geographic atrophy. Proc Natl Acad Sci U S A 2020;117:9952-63. [Crossref] [PubMed]
- A Study Assessing the Safety, Tolerability, and Efficacy of RG6147 in Participants With Geographic Atrophy Secondary to Age-Related Macular Degeneration (AMD) (GALLEGO) Available online: https://clinicaltrials.gov/ct2/show/NCT03972709
- Wong WT, Kam W, Cunningham D, et al. Treatment of geographic atrophy by the topical administration of OT-551: results of a phase II clinical trial. Invest Ophthalmol Vis Sci 2010;51:6131-9. [Crossref] [PubMed]
- Sternberg P, Rosenfeld PJ, Slakter JS, et al. Topical OT-551 for Treating Geographic Atrophy: Phase II Results. Invest Ophthalmol Visual Sci 2010;51:6416.
- Bandello F, Sacconi R, Querques L, et al. Recent advances in the management of dry age-related macular degeneration: A review. F1000Res 2017;6:245. [Crossref] [PubMed]
- Phase II/III Study of the Efficacy and Safety of MacuCLEAR MC-1101 in Treating DryAge-Related Macular Degeneration (McCP2/3) Available online: https://clinicaltrials.gov/ct2/show/NCT02127463
- da Cruz L, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 2018;36:328-37. [Crossref] [PubMed]
- Kashani AH, Uang J, Mert M, et al. Surgical Method for Implantation of a Biosynthetic Retinal Pigment Epithelium Monolayer for Geographic Atrophy: Experience from a Phase 1/2a Study. Ophthalmol Retina 2020;4:264-73. [Crossref] [PubMed]
- Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med 2018;10:eaao4097. [Crossref] [PubMed]
- Sharma R, Khristov V, Rising A, et al. Clinical-grade stem cell–derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med 2019;11:eaat5580. [Crossref] [PubMed]
- Stern JH, Tian Y, Funderburgh J, et al. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018;22:834-49. [Crossref] [PubMed]
- Qiu TG. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells (MA09-hRPE) in macular degeneration. NPJ Regen Med 2019;4:19. [Crossref] [PubMed]
- Sloan FA, Hanrahan BW. The Effects of Technological Advances on Outcomes for Elderly Persons With Exudative Age-related Macular Degeneration. JAMA Ophthalmol 2014;132:456-63. [Crossref] [PubMed]
- Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in denmark: year 2000 to 2010. Am J Ophthalmol 2012;153:209-13.e2. [Crossref] [PubMed]
Cite this article as: Israilevich R, Mahmoudzadeh R, Salabati M, Xu D. Narrative review-drug delivery in age-related macular degeneration. Ann Eye Sci 2021;6:33.


