
Periocular basal cell carcinoma—current treatment concepts
Basal cell carcinoma (BCC) is by far the most common human skin cancer (1,2). In Caucasians, BCCs account for around 90% of periocular malignancies, while squamous cell carcinoma (SCC), sebaceous gland carcinoma, melanoma, and some rarer tumors the remaining 10% (3). In Germany, the annual incidence is estimated at up to 200 per 100,000 inhabitants (4). The average age of the patients is over 60 years, which is in accordance with the rapidly increasing incidence in an aging society (4). BCC develops as epithelial-basaloid neoplasia and is accompanied by mostly infiltrating and destructive growth (5,6). Distant metastasis is rare. Increased risk of suffering from BCC is associated with exposure to ultraviolet (UV)-B, hereditary diseases [e.g., Gorlin-Goltz syndrome and Xeroderma pigmentosum (XP)], ionizing radiation and long-term immunosuppression, among others (7).
However, periocular BCCs are usually neglected due to their slow and painless growth, unless presenting complaints, e.g., large size, bleeding, recurrent infections of the tumor, or secondary symptoms resulted from adjacent structures involvement such as epiphora, limited eye globe motility, as well as globe displacement (8). Furthermore, although the tumor can usually be cured with local excision, local recurrence can occur in up to 20% of eyelid BCC cases (9). Recurrent BCCs of the eyelid show a poorer overall prognosis than the primary ones (10). Besides, the management of advanced diseases, such as orbital or intracranial invasion as well as metastatic lesions, is challenging and often involves a multidisciplinary approach. Therefore, we review recent research progress of pathogenesis, clinical presentation, and therapeutics of periocular BCCs.
Molecular pathogenesis
Multi-step UV-induced carcinogenesis model
Photocarcinogenesis defines the multi-stage development of skin cancers resulted from electromagnetic waves of the optical spectrum, which involves the activation of oncogenes and suppression of tumor suppressor genes. That is influenced by dose, exposure time, and wavelength. The optical spectrum belongs to the non-ionizing part of the electromagnetic spectrum and consists of UV radiation (100–400 nm), visible light (400–760 nm), and infrared (IR) radiation (760 nm–1 mm). Among them, UV radiation is deemed the primary cause of photocarcinogenesis in the development of skin BCCs and SCCs (11). According to the multi-step photocarcinogenesis model, BCCs have a very high load of UV-induced gene mutations (driver mutations), which lead to the failure of crucial cellular signaling pathways. With the help of next-generation sequencing (NGS), three-quarters of all mutations in BCCs are shown UV-induced (12).
Deoxyribonucleic acid (DNA) is a scaffold for the assembly of chromophores that absorbs UV radiations (13). UVB radiation is directly absorbed by the DNA, which leads to the formation of DNA photoproducts, i.e., cyclobutane pyrimidine dimers (CPDs) and 6-pyrimidine 4-pyrimidone dimers (6-4 PPs). These photoproducts lead to “bulky lesions” in the DNA. These lesions represent a distortion of the DNA backbone due to mismatching of bases or photoproducts and prevent transcription and replication by blocking the polymerases. If these lesions are not repaired before the S phase of the cell cycle, characteristic C to T mutations may occur. These mutations are based on the rule that the loss of a proofreading function due to mutations causes DNA polymerase η to complement lesions such as CPDs by two adenines on the opposite strand (14-16).
Only nucleotide excision and repair counteracts these mutations. Defects in this repair route cause the rare autosomal recessive disease XP (17). XP impressively demonstrates the clinical consequences of a lack of repair of UV-induced DNA damage and the accumulation of DNA mutations, in which BCC and other UV-induced skin tumors develop in childhood with the initial diagnosis at a median age of 8 years.
Genetic predisposition
Although most BCCs are deficient in any pre-existing genetic background, several tumor suppressor or promoter genes have been found be involved in BCC pathogenesis, e.g., components of the Sonic Hedgehog pathway (PTCH1 in 73% and SMO in 20%), the TP53 tumor suppressor gene (in 61%), and members of the RAS family (13,18). Eight-five percent of BCCs carry activating mutations in the Hedgehog signaling pathway, which plays a significant role during embryonic development (19). The improper activation of the Hedgehog signaling pathway appears to be the key component pathway in the development of neoplasia in BCCs (20,21).
Furthermore, other mutations also occur in other critical cellular signaling pathways involved in the carcinogenesis of BCCs. Eight-five percent of other “driver mutations” were found in other tumor suppressor or tumor promoter genes (13). The hippo-YAP (Yes-associated protein) signaling pathway is one of these genes (19). This signaling pathway regulates tissue homeostasis and is active in growth control and apoptosis regulation (22). Genetic profiling using the NGS identified mutations in the hippo-YAP signaling pathway genes LATS1 (16%), LATS2 (12%) and PTPN14 (23%). Other mutated genes that are significantly associated with the development of BCCs include MYCN (30%), PPP6C (15%), STK19 (10%), RB1 (8%), FBXW7 (5%), and ERBB2 (4%) (19).
Epigenetic changes
Heritable genomic modifications in eukaryotic cells may be produced without alterations in the genomic DNA sequence, which is known as epigenetics. Epigenetic alterations are mainly comprised of CpG Island Methylation (CIM), histone methylation and acetylation, and gene regulations mediated by miRNAs. DNA methylation is one of the most essential mechanisms for regulating gene expression (18). Heitzer et al. presented the PTCH promoter to be hypermethylated in a few cases and proposed that this methylation might only play a minor part in BCC carcinogenesis (23), while Goldberg et al. found the hypomethylated FHIT promoter (24). Darr et al. investigated metastatic BCCs in comparison to the non-metastatic ones and found hypomethylation at MYCL2 (25). Furthermore, among of the extensive modifications of histone N-terminal tail regions, methylation and acetylation are the most well-studied ones. EZH2, a histone methyltransferase, was found upregulated in aggressive BCCs, while H3K27me3 and 5hmC were indicated to be upregulated in more benign phenotypes (26). The upregulated levels of different genes might be applied to discriminate BCCs from benign skin diseases. In addition, mature miRNAs may target specific mRNAs and degrade them or inhibit their translation into proteins. A number of potential miRNA markers for BCCs have been investigated in numerous studies. Various upregulated miRNAs were identified, e.g., Hsa-miR-223-3p and Hsa-miR-197-3p, among others (27). miR-203 is specifically expressed in the epidermis and create an inhibitory loop of miR-203 c-JUN (18,28). It was found downregulated in BCC cases, and its therapeutic potential for BCCs has been demonstrated (29).
Clinical classification
Sporadic BCC
BCCs are classified clinically based on the types of growth (13,30). The nodular type represents about 60% of all BCCs. It is characterized by the triad of pearl cord-like margins, central ulceration, and telangiectases over the margins (Figure 1). Histologically there is a palisade-like arrangement of the tumor cells on edge with a dense tumor stroma that delimits the tumor. The BCC cells have prominent nuclei rich in chromatin and occasionally mitoses (13,31).
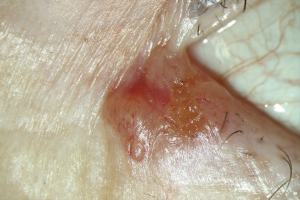
The multicenter, superficial type accounts for 25% of all BCCs. Clinically it is characterized by large (usually several centimeters), red, and eczema-like plaques. Within the plaques, there are histologically multiple foci of the tumor that penetrate the epidermis and possibly the uppermost dermis. Furthermore, the morphea-like BCC is the most problematically approachable in therapeutics, which is rare in 2% of all BCCs (32). Herein, the tumor boundaries can often only be histologically delineated by elongated tumor cones that infiltrate the surrounding tissues. Additionally, other rare and histologically distinguishable BCC types include basosquamous tumor, pigmented BCC, metatypical BCC with metastatic potential, rodent ulcer or ulcer terebrans, and Pinkus tumor (fibroepithelioma) or collision tumor.
Gorlin-Goltz syndrome
The hereditary multi-system Gorlin-Goltz syndrome (BCC syndrome or basal cell nevus syndrome) is characterized by the appearance of many skin BCCs at a young age. Other symptoms include jaw cysts, rib anomalies, and calcification of the falx cerebri (13). Medulloblastoma occurs in 5–10% of the patients in childhood. In general, characteristic malformations are in the skeletal system, central nervous system, urogenital system, and the heart. The incidence of BCC syndrome is 1:56,000 (33). The mode of inheritance is autosomal dominant, and the heterozygous carriers of an activating mutation of the Hedgehog signal pathway are affected. The majority of mutations are found in the PTCH1 gene (33). Almost half of those affected have a negative family history—an indication of the high spontaneous new mutation rate. BCCs occur when the normal allele mutated by a second somatic mutation event (“the second hit”; “loss of heterozygosity”) in addition to the heterozygous germline mutation in a keratinocyte. Therefore, if there is evidence of Gorlin-Goltz syndrome, sun protection should be practiced as early as possible, and regular skin cancer screening examinations should be carried out at 3 to 6 months intervals (13). Ionizing radiation also triggers somatic mutations in these patients. Gorlin-Goltz syndrome patients usually develop massive BCCs in the skin region where they had radiation therapy. Therefore, ionizing radiation should be avoided diagnostically and therapeutically, while magnetic resonance imaging examinations should be preferred (33).
Clinical tumor-node-metastasis (TNM) staging of eyelid BCC
Accurate staging of a skin cancer is fundamental for optimal patient management. Cancer stage, termed TNM stage or stage group, takes account of tumor characteristics (T, by physical examination), regional spread to lymph node(s) (N, by physical examination), and metastasis of distant organs (M, by physical examination and imaging). The regional lymph nodes involve the preauricular, submandibular, and cervical lymph nodes. The latest 8th edition TNM classification system of malignant tumors (TNM8) was published in 2017 by the Union for International Cancer Control, which formed the foundation for handling and reporting skin cancer cases (34).
The latest 8th edition TNM classification system of non-melanoma eyelid skin cancers (NMSC, typically including the basal cell, squamous cell, and sebaceous carcinoma) are displayed as follows. As for T (primary tumor), T0 indicates no evidence of primary tumor, and Tis carcinoma in situ. T1–T3 categories are stratified at ≤20, >20 to 40 and >40 mm in maximum tumor dimensions, respectively. Subdivisions of a and b are defined as with or without tarsal plate or eyelid margin invasion, and c is deemed the involvement of full thickness of eyelid. Furthermore, T4 is defined by the invasion of adjacent ocular, orbital, or facial structures in any sizes of tumor. If the eyelid BCC invades ocular or intraorbital structures, the subdivision of T4a is defined. T4b is deemed by the presence of bony walls of orbit erosion, paranasal sinuses extension, or lacrimal sac/nasolacrimal duct or brain invasion (34).
With regard to N (regional lymph nodes), Nx indicates unevaluable regional lymph nodes, N0 no evidence of lymph node involvement, N1 metastasis in a single ipsilateral regional node with a greatest dimension of 3 cm or less, and N2 metastasis in a single ipsilateral regional node with a greatest dimension of more than 3 cm or in bilateral or contralateral lymph nodes. For M (distant metastasis), M0 is defined by no distant metastasis, and M1 distant metastasis (34).
The above mentioned TNM system has been applied to describe and record the anatomical extent of tumor. Stage and prognostic groups are adopted to ensure, as far as possible, the homogeneity of each group with regard of survival and the distinction of these groups in respect of the survival rates by condensing these TNM categories into groups. In general, carcinoma only in situ (Tis) is designated as stage 0; location at the eyelid as stages I and II, i.e., T1 as IA, T2a as IB, T2b, T2c, and T3 as IIA, and T4 as IIB; extension to regional lymph nodes, in M0 and any T, as stage III, i.e., N1 as IIIA, and N2 as IIIB; and distant metastasis (M1), in any T and N, as stage IV (34).
In addition, the prognostic factors for survival for eyelid NMSC are divided in to essential, additional, and new and promising categories according to the ninth edition of the UICC Manual of Clinical Oncology (35). In essential factors, worse prognosis is indicated by the presence of orbit or sinus invasion, immunosuppression of host, preauricular and/or cervical lymph node involvement, or systemic metastasis at presentation. In additional factors, eyelid BCC and SCC have a better prognosis than sebaceous tumors, and the nodular BCC has a better prognosis than morpheaform ones. With regard to new and promising factors, improvements in local control relate to less systemic recurrence.
Surgical resection
Excision with histologic margin assessment is the standard treatment modality for periocular tumors. After successful tumor excision, a histological examination should always be carried out to confirm the diagnosis and to check the excision margin with the determination of the resection status. The safety distance is difficult to define for BCCs of the eyelids since every millimeter of healthy tissue would be decisive for later functional reconstruction. The surgical resection should always excise as much tissue as necessary to achieve an R0 resection. However, as little healthy tissue as possible should be removed to ensure the best possible reconstruction with excellent functional and cosmetic outcomes (36).
Intraoperative margin control (IOMC) examines the tumor and its margins before reconstruction and is increasingly widely utilized. It primarily involves Mohs micrographic surgery (MMS), fast frozen sections (FFS), and fast paraffin (FP). Phan et al. performed a meta-analysis comparing recurrence rates of MMS, intraoperative FSE controlled excision, and wide local excision (WLE) with predetermined margins as well as paraffin section evaluation (37). The pooled recurrence rates for periocular BCCs following MMS, FSE, and WLE were 2.9% (95% CI, 1.9–4.4%, an average follow-up of 48.8±14.9 months), 1.9% (95% CI, 1.9–2.4%, an average follow-up of 70.7±48.0 months), and 5.9% (95% CI, 3.9–8.9%, an average follow-up of 49.2±29.3 months), respectively. Meta-regression analysis illustrated an insignificantly similar recurrence rate for MMS and FFS (P>0.05) and a significantly lower one in comparison to WLE without FSE.
Frederic Mohs first reported MMS in 1938. It has been adopted as the gold standard management modality for certain BCCs (37) and recommended for those in high-risk areas (e.g., area H including the eyelids), with large size (over 2 cm), with aggressive histopathological subtype, with indistinct clinical margins, and residual as well as recurrent BCCs by The British Association of Dermatology and Appropriate use criteria (AUC) from the United States of America (7,38). After debulking the clinically apparent tumor plus a narrow margin, the excised tissues are immediately, without immersed in formalin, provided to the pathologist for assessment. After macroscopic assessment by the pathologist, the sample is cut on the cryostat and stained in a rapid process for horizontal or en face frozen section histological evaluation of the entire periphery and under the surface. Results are communicated to the surgeon by telephone or online 15–40 minutes later (depending on the type and quantity of the samples). If the pathologist reports tumor-free resection margins on all margins (pR0 resection), the ophthalmic plastic reconstruction would be started directly. In the case of a pR1 or pR2 status (histologically or macroscopically not tumor-free resection margins), resection should be carried out again. Afterward, another frozen section histological evaluation of the excised sample should be carried out, which should be done until a pR0 status is achieved.
FFS is undertaken by freezing the sample and subsequently histologically evaluating vertical (bread-loaf) sections. Freezing sections show a loss of quality compared to conventional histology specimens so that a final diagnosis only follows a conventional tissue processing with formalin fixation and regular staining. Compared to FP, MMS and FFS have the great advantage that tumor excision and oculoplastic reconstruction can be carried out in one session (39). FP, or rush paraffin assessment, also evaluates vertical (bread-loaf) sections, but the sample is fixed by formalin and processed in a unique, time-reduced process. The processing time is usually about 3–4 hours. A result is usually available the next day. The advantage of FP is a better and safer assessment of the excision margin due to less quality loss. Furthermore, a first histological diagnosis is already available at the time of the reconstruction. Its disadvantage is the multi-stage process. The patients are operated on in at least 2 sessions on 2 different days. If a re-excision is necessary, the number of sessions would increase again. However, it must be noted that there is no randomized study that compares the FFS with the FP for section margin diagnosis in periocular BCCs concerning recurrence rates (39).
A postoperative histopathological examination is essential not only to confirm the diagnosis and determine the resection status but also to identify infiltrating growing subtypes, as this also influences postoperative follow-up care and the prognosis (6,40,41). To further differentiate from other tumors, such as SCC, further immunohistochemical examinations such as BerEP4 [synonym epithelial cell adhesion molecule (EpCAM)] and epithelial membrane antigen (EMA) can also be performed after the histological examination (41).
Oculoplastic reconstruction
After a successful pR0 resection, specific conditions of the patients require different oculoplastic reconstruction strategies. The selection of an appropriate technique not only depends on the vertical and horizontal defect size, the location of the defect, or a potential eyelid margin involvement, but also significantly on the age of the patient, the available tissue (e.g., excess skin), the patient’s wish, and in particular on the experience of the surgeon (36,39,41). Therefore, every oculoplastic surgeon should master a wide range of reconstructive techniques to restore the anatomical relationships with the anterior and posterior eyelid lamellae. The general rule is that only one flap and no second free graft may be used for the reconstruction of the anterior lamella, and only one free graft for the posterior lamella. In the case of a reconstruction of the posterior eyelid lamella with a free graft, the anterior eyelid lamella must then be provided with a flap rather than receiving a second free graft. The graft tissues can originate from the ipsilateral or contralateral eyelid or other parts of the body. Besides, artificial or even foreign support tissue can be transplanted (36).
The eyelid consists of anterior lamella (i.e., skin and orbicularis oculi muscle) and posterior lamella (i.e., tarsus and conjunctiva). When repairing full-thickness defects, both the anterior and posterior lamellae should be reconstructed. Small full-thickness marginal eyelid defects ≤25% of the width of the eyelid can be directly closed in two layers, i.e., the tarsus and the skin. A horizontal mattress is recommended to close the eyelid margin, helping wound edge eversion and recovery without a notch. For defects involving 25–50% of the width of the eyelid, lateral canthotomy and cantholysis can be undertaken. The lateral eyelid is then pulled medially to cover the defect. Besides, a periosteal flap with the pedicle to the lateral orbital rim may enhance the posterior lamella and facilitate the repairing of more substantial defects. For medium-sized defects involving 33–66% or up to 75% of the width of the upper and lower eyelids, a Tenzel semicircular musculocutaneous rotation flap may be utilized to reconstruct the anterior lamella. For extensive defects, even up to 100% of the lower eyelid, a Hughes tarsoconjunctival flap or midface lift may be performed, and free graft is also an option for reconstructing both lamellae (42).
Tenzel semicircular myocutaneous rotation flap
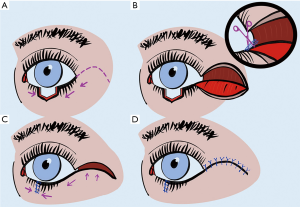
This technique is suitable for reconstructions of the upper and lower eyelids with full-thickness defects too extensive to close directly, but still less than 75% of eyelid width (36). It may also be carried out in patients refuse to sew his/her eye for 3 to 4 weeks with a Hughes procedure. The Tenzel flap is a semicircular myocutaneous flap with a semicircular incision at the lateral canthus and separation of skin and orbicularis, as shown in Figure 2. A complete lateral cantholysis is also required to create this flap. Subsequently, the flap is advanced medially into position, and the eyelid defect is closed in the same fashion as used with wedge resection. The lateral defect resulting from the flap preparation is then closed with interrupted sutures (36).
Cutler-Beard bridge flap
Cutler-Beard bridge flap is one of the crucial techniques for a total or near-total upper eyelid defect (more than 75% in size), even though extensive defects of the upper eyelid are a typical indication for a Tenzel semicircular rotation flap (36). This full-thickness flap is a two-stage eyelid sharing procedure, as shown in Figure 3.

In the first stage, a rectangular advancement flap was made with a full-thickness horizontal blepharotomy 4 to 5 mm below the lower eyelid lash line and then a vertical blepharotomy extending downward to the inferior fornix. Subsequently, the myocutaneous conjunctival advancement flap is transposed beneath the marginal lower eyelid bridge and sutured to corresponding layers in the upper eyelid defect. Care should be taken to maintain an intact lower eyelid bridge with an intact tarsus and the medial and lateral palpebral arteries, which reduces the risk of later complications such as lower eyelid necrosis. The flap does not involve the tarsus. As a result, the eyelid margin may be unstable and predisposed to entropion when the defect is more substantial than 75% in size. It can be addressed by placing a posterior lamellar graft between the conjunctiva and orbicularis muscle, which is attached to the levator aponeurosis to approximate the tarsus. This tissue can originate from the donor sclera, autogenous auricular cartilage (43), or the tarsus of the contralateral eyelid (36).
In the second stage, the full-thickness pedicle is cut 2 mm below the desired position of upper eyelid margin (to account for tissue contraction) and re-fixed to the marginal bridge of the lower eyelid in the correct layer after 4 to 6 weeks (36,39). If necessary, the conjunctiva can also be sewn to the skin via the new upper eyelid edge. Although eyelashes are lacking in the newly reconstructed upper eyelid, the procedure provides an excellent functional and cosmetic result. Significant contraindications for a Cutler-Beard bridge flap are better (or only) seeing eye on the affected side, or the patient’s refusing a second operation.
Hughes flap
Tarsoconjunctival advancement flap (i.e., Hughes flap) is a pedicled flap of the conjunctiva and tarsus of the upper eyelid, as shown in Figure 4. It is used for reconstructing extensive full-thickness defects, even up to 100% of the lower eyelid, especially in the case of central lower eyelid defects with medial and lateral residual tarsus (36). Since excellent cosmetic and functional outcomes are achieved, it has been one of the most important basic techniques in oculoplastic surgery.
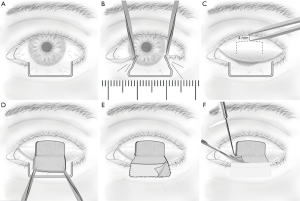
The classical Hughes procedure mainly involves an incision plane starting at the grey line of the upper eyelid margin and a dissection plane splitting the eyelid over the entire tarsal height and leaving the levator and Müller’s muscle complex attached to the tarsus (45). Subsequently, the flap is sutured tarsus to tarsus into the defect to recreate the posterior lamella of the lower eyelid, and then either a free skin graft or a skin-muscle advancement flap is performed to reconstruct the anterior lamella of the lower eyelid (44). According to different studies, flap division is undertaken 2–8 weeks after the Hughes procedure under local anesthesia (44,46,47). Donor-site complications, i.e., entropion, trichiasis, damage to the eyelash root bulbs, and upper eyelid retraction, have been attributed to both of the planes (45). Currently, most authors tend to completely separate the levator and Müller’s muscle from the superior tarsal border during flap preparation, which leaves a delicate pedicle only with blood supply from the thin conjunctiva and may result in necrosis of the flap (48).
We are performing a modified Hughes procedure (44). In this procedure, the incision plane spares 4 mm of the marginal tarsus. Subsequently, the dissection plane separates the entire levator from the tarsus but leaves most of Müller’s muscle fibers attached to the superior tarsal margin (44). The modified Hughes procedure has been demonstrated to be a well-suited technique for reconstructing lower eyelid defects involving up to 100% of the horizontal eyelid length. The Hughes flap with Müller’s muscle attached is more robust and thicker. It seems to reduce the risk of premature flap dehiscence without raising the occurrence of upper eyelid retractions in turn.
Alternative therapies
In addition to histologically margin-controlled excision surgery, the gold standard for managing BCC, alternative treatments have been used and accordingly addressed in the current new German S2k guidelines (33) and the European Consensus-Based Interdisciplinary Guidelines (49). The alternative treatment options for BCC can easily be divided into three groups due to their range of uses, i.e., radiation and new systemic therapies, topical therapies, and prophylactic approaches.
First, radiation and new systemic therapies such as the Hedgehog signaling pathway inhibitors (i.e., Hedgehog inhibitors), immune checkpoint inhibitors, and electrochemotherapy are used when the BCC is metastatic or locally advanced (definition of the guideline: “Tumors that require an interdisciplinary therapy concept due to expansion and in particular destructive deep growth”) (4,33). These tumors, in particular, can often no longer be treated surgically. A patient’s refusal of surgery is also a not inconsiderable reason to use an alternative therapy. The radiation therapy is no longer maintained as second-line therapy in the new national guideline so that a tumor board can more freely determine individual therapy. However, the tumor board is still free to recommend surgical or other concepts. In summary, for advanced BCCs, radiation therapy or systemic therapy are possible following the interdisciplinary tumor board of the respective hospital.
Second, topical therapies can be used as an alternative to histologically margin-controlled excision for small BCCs (2 mm in thickness as a reference value due to the lack of evidence) with a low risk of recurrence (50). Factors increasing the risk of recurrence are as follows: a horizontal tumor diameter of >6 mm in the periorbital area, difficult-to-define boundary, local recurrence, histological subtypes (e.g., sclerodermiform, infiltrative, metatypical, or micronodular growth), a tumor on adequately irradiated skin, and perineural growth. BCCs with a low risk of recurrence provide the position for topical procedures such as imiquimod therapy (toll-like receptor 7 agonist), mitosis inhibitor 5-fluorouracil (5-FU), photodynamic therapy with 5-aminolevulinic acid (5-ALA) or its ester methyl aminolevulinate (MAL), cryotherapy, and laser therapy. Also, semi-surgical procedures such as curettage and flat excision are still part of treatment concepts for small BCCs. Other topical procedures such as treatment with ingenol mebutate and diclofenac are currently not recommended for the treatment of BCC due to the lack of evidence-based data. However, the main point of criticism, in contrast to surgery, is always the lack of success monitoring, i.e., a R0 resection. Therefore, the authors almost always recommend, especially in the eye area, to offer surgery to patients in these cases (50).
Third, prophylactic approaches to BCC are more or less recommended (50). Nicotinamide can be used in patients with a history of BCC. Whereas, retinoids (cell cycle inhibitors) have no significant effect on the prevention of relapse in BCC, but with side effects such as headache, muscle pain, and noticeable teratogenicity. As in primary therapy for BCC, there is no reliable evidence for cyclooxygenase 2 (COX2) inhibitors in prophylaxis after an initial event.
Radiation therapy
Other indications for radiotherapy, not mentioned above, may include the patient’s intolerance to anesthesia for extensive surgical intervention and the desire for curative BCC treatment with organ preservation or with the best possible protection of the patient’s physiognomy. The cure rates of various forms of radiation (various fractional percutaneous forms of radiation or brachytherapy) are roughly comparable to conventional surgery (51-54). However, difficulties in assessing the skin after possible recurrences often arise due to the scarring after radiation.
To date, in the only comparative, randomized study, a superiority of histologically controlled surgery (99% freedom from recurrence) compared to radiation therapy (92.5% freedom from recurrence) was demonstrated within a follow-up period of 4 years (55). Gorlin-Goltz syndrome is a relative contraindication to radiation, as an increased secondary tumor rate or a large number of new BCCs were found during follow-up (40). Particularly in the case of a residual tumor after the surgical excision of a BCC, the use of radiation therapy can be a sensible option (53). The effectiveness in “high-risk” BCCs has also been demonstrated (54). For brachytherapy, the success rate was 92.5% for primary untreated BCC and 88% for R1 or R2 surgically excised BCCs (56).
In the context of radiation treatment, all radiation techniques have been used accordingly. The goal is to achieve a proper dose distribution in all skin layers. The radiation planning, therefore, encloses the tumor region with an oncological adequate safety margin, usually 0.5–1.5 cm. The total dose is between 50 and 74 Gy, depending on the tumor mass (52-54). Due to the proximity to the radiation-sensitive organs, low single doses (e.g., 1.8 Gy) are often used (40,57,58).
Proper periorbital radiation planning includes reaching an effective dose on the entire BCC lesion while protecting the radiation-sensitive eye structures such as the cornea, lens, retina, optic nerve, and lacrimal gland (40,57,58). Nevertheless, radiation-induced side effects can occur in different forms as follows: eyelash loss, sicca symptoms, corneal surface lesions such as conjunctivization, and radiation-induced cataracts, among others. Radiation retinopathy and opticopathy are rare in the treatment of BCC and should generally be excluded by proper radiation planning. A collaboration between the ophthalmologist and the radiation therapist helps to design the spatial concept of radiation to minimize side effects. Especially with percutaneous radiation, the motility control of the eye is essential. It is usually achieved with a camera, on which the patient fixes with the eye during the radiation.
The risk of the radiation-related secondary tumors remains undisputed, with the latency period of at least 10 years. Therefore, the age of the patient can be an essential criterion when deciding on radiation therapy (59,60).
Systemic therapy
Hedgehog inhibitors
Activation of the Hedgehog signal pathway is an essential step in the pathogenesis of BCCs. It has been detected in over 85% of all BCCs (19). Therefore, Hedgehog inhibitors have been developed to act on this signal pathway. Currently, Vismodegib (Erivedge, Roche, Basel, Switzerland) has been approved for the indication of adult patients with metastatic or locally advanced BCC inappropriate for surgery or radiotherapy based on the ERIVANCE study (Efficancy and Safety of Vismodegib in Advanced Basal Cell Carcinoma) (61).
A total of 71 patients with inoperable, locally advanced lesions, and 33 patients with metastatic BCCs were included in this study (61). Gorlin-Goltz syndrome patients were also included in advanced BCCs. Patients received 150 mg of Vismodegib until either tumor progression, medication-related toxic side effects, or cancellation due to the patient’s reasons. The therapy responded to 30% of all patients with metastatic BCC and 60% with localized lesions. However, However, the high dropout rate was found due to apparent side effects (63% muscle spasms, 61% alopecia, 54% taste disorders, 32% weight loss, 28% asthenia, 22% taste loss, 17% diarrhea, 16% fatigue, and 16% nausea) (50,61,62). One-year data confirmed positive treatment results and significant side effects. Studies on Gorlin-Goltz syndrome have shown similar success (63). Further studies on the effectiveness and safety of Vismodegib (STEVIE, NICCI, and MIKIE study) showed comparable results (64). Due to the teratogenic effects of the Hedgehog inhibitors, women and men must use contraceptive measures during and after treatment. Since it is assumed that almost every BCC has a Hedgehog signaling pathway mutation that is relevant for therapy, deliberately no termination criteria were formulated for this chemotherapy. However, the doctor should pay attention to whether the success of the therapy outweighs the side effects for the patients.
Sonidegib (Odomzo, Sun Pharmaceutical, Mumbai, India) is another Hedgehog inhibitor for BCC approved based on the BOLT study (treatment with two different doses of sonidegib in patients with locally advanced or metastatic BCC) (65). Patients with local BCCs that could not be treated or with metastatic BCCs were treated with 200 or 800 mg of Sonidegib, respectively (65). Due to the better response at the lower dose (56% to 45% of 800 mg group), 200 mg daily was approved with a similar side effect profile to Vismodegib. Metastatic BCC was not included in the indication range due to a low response (65).
Initial treatment successes with the Hedgehog inhibitor vismodegib have been demonstrated for periorbital BCC (66-68). However, in an observational study with seven patients, a new SCC of the skin developed during treatment in two patients, although it is unclear whether there is a causal relationship to Hedgehog inhibitor therapy (69). Two other studies of patients with periorbital BCCs showed progressive disease after a complete or partial remission due to vismodegib (67,68). Furthermore, a case report of a 6-month treatment for BCC with Vismodegib reported a histological examination mapping over the entire back of the scalp. It showed a massive spread of tumor cells beyond the original tumor boundaries (70), which is followed by extensive resection. Treatment with Vismodegib can be of benefit in the periorbital area, provided that improved success control of the tumor reduces recurrence rates. It remains to be seen whether the tumor cells develop resistance to the active substance of the drug. Further data on the adjuvant, especially on the neoadjuvant in combination with surgery, is eagerly awaited, which may make it possible to maintain the eyes even in intractable situations.
Immune checkpoint inhibitors
The immune checkpoint inhibitors, especially the programmed cell death protein 1 (PD-1) antibody, have almost revolutionized cancer treatment in the past 2 to 3 years. Initially, the value of PD-1 antibodies has been demonstrated in small cell bronchial carcinoma (71,72). Similar successes have also been seen in cutaneous melanoma therapy (72).
The groundbreaking success of immune checkpoint inhibitors is underlined by the awarding of the Nobel Prize in Medicine for their discovery in 2018. Immune checkpoints represent antigenic barriers for the immune system and prevent the body’s cells from being recognized by immune-competent cells. Cancer cells utilize this autoimmune protective function to remain undetected by the immune system. Immune checkpoint inhibitors override these antigen barriers so that immune-competent cells such as T-lymphocytes can recognize and fight the tumor cells. This therapy is particularly useful when a large number of tumor-related mutations occurred in a cancer cell, as is the case with BCC. Various case reports have shown the initial successes of PD-1 antibodies in locally advanced or metastatic BCCs (73-76).
Combination of different systematic therapies
New data and case reports on combined treatment concepts with a Hedgehog pathway inhibitor and an immune checkpoint inhibitor are also eagerly awaited. It would be of interest to investigate whether the therapy successes add up and to what extent the side effect profile remains reasonable for the patient.
Topical therapy
Sometimes topical therapies for BCC are used primarily in dermatology. The use of these alternative therapies is not very common in ophthalmology. In most cases, the patient is initially offered the histologically controlled excision periorbitally. These topical therapies for periorbital BCCs can only be offered if the patient has severe reservations about surgery, then preferably in collaboration with a colleague with experience in this field.
Imiquimod therapy
Imiquimod is an immune response modifier that acts as a toll-like receptor 7 agonist. Its initial treatment spectrum is revealed as antivirals. Five percent cream 5 days a week for 6 weeks is recommended. The European approval for use in BCC is an indication spectrum of <2 cm in tumor diameter (77). Comparative studies showed inferiority to surgery in terms of freedom from recurrence, but superiority to 5-FU therapy and photodynamic therapy with MAL (78,79). Side effects include redness, swelling, desquamation, blistering, and pain (77). Flu-like symptoms with local lymph node swellings can also occur (77).
5-FU
5-FU is a mitosis inhibitor well known in ophthalmology and currently used after filtering glaucoma surgery. It is applied to the skin in a 5% concentration twice a day for 4 weeks (80). Side effects can also include redness, swelling, desquamation, blistering, and pain.
Photodynamic therapy
5-ALA or its ester methyl-aminolevulinate (MAL, only approved for BCC in Germany) are emulsions activated on the skin with a 635-nm red light illuminator. Protoporphyrin IX, which is generated in tumor tissue from 5-ALA or MAL, is activated and then destroys the tumor cell by generating singlet oxygen formation. In terms of freedom from recurrence, this treatment is inferior to histologically controlled surgery and imiquimod therapy (78,80). Side effects—in addition to the pain during treatment—are initial erythema as well as erosion and crust formation a few weeks after the treatment.
Cryotherapy
Cryotherapy with liquid nitrogen in contact or spray processes leads to icing at –196 °C. A comparative study showed obvious inferiority to radiation therapy (81). Likewise, scars usually occur after this therapy, which can initially mask recurrence.
Laser therapy
In laser treatment for BCCs, ablative procedures are distinguished from non-ablative procedures (50). In the ablative procedure, superficial skin tumor lesions are removed using CO2 or Er: YAG lasers. In contrast, the tumor vessels of BCCs are obliterated in the non-ablative procedures. However, due to the tendency of BCCs to expand in depth, close monitoring after treatment is essential.
Ingenol mebutate
Ingenol mebutate, a substance found in garden milkweed, is an inducer of cell death and has been used for keratosis primarily. A phase III study is currently missing, which would further confirm the good phase II data in BCC (82). Side effects include redness, swelling, scaling, blistering, and pain.
Diclofenac
Because of the possible role of COX2 in the development of BCC, an approach with the COX2 inhibitor diclofenac was pursued. An inhibitory effect on superficial BCCs was seen in a phase II study, whereas no effect was seen in nodular BCCs (83). Therefore, treatment with a COX2 inhibitor cannot currently be recommended.
Prophylactic therapy
Nicotinamide (vitamin B3) is an active ingredient that helps the organism to repair DNA breaks in cells and thus to counteract UV damage. Data from the Nurses’ Health Study and the Health Professionals Follow-Up Study, which documented the intake of nicotinamide, were evaluated. The administration of 500 mg nicotinamide twice daily could reduce the development of new BCCs by 20% (95% CI, –6% to 39%) than that with placebo in patients who had undergone ≥2 non-melanoma skin cancers in the previous 5 years (84). However, no prophylactic effect of nicotinamide was seen for BCC development as a primary skin tumor (85).
In contrast to SCC, retinoids, a cell cycle inhibitor, could hardly be found to have a prophylactic effect for BCC. Due to the side effect profile with headache, muscle pain, Sicca symptoms, arthralgia, exhaustion, depression, and teratogenicity, the current intake is not recommended (86).
Conclusions
In summary, in most cases, the highest priority for periocular BCCs is complete, histologically margin-controlled tumor excision (pR0). The intraoperative histopathological examination for margin control can be carried out using MMS, FFS, or FP. The reconstruction of the eyelid is essential in consideration of the peculiarity of the eyelid, especially its protective function for the globe. A variety of reconstruction methods enable individual adaption as well as cosmetic and functional coverage of eyelid defects in most cases. Postoperative regular tumor follow-up is also essential for subsequent local recurrence. Furthermore, alternative treatment options are also available for inoperable patients with BCCs. In particular, the introduction of Hedgehog inhibitors (Vismodegib) as novel oral treatment for advanced BCCs has added a new treatment option to previous therapies. BCCs also have about 85% mutations in other genes that drive the development of the carcinoma. These are also suitable for future targeted therapy, which, however, still awaits further development. Due to the high mutation load, immunotherapy with checkpoint inhibitors is effective in addition to Hedgehog inhibitors. Prevention of BCC can be an option in recurrence cases. Sun protection and taking vitamin B3 (nicotinamide) reduce the risk of BCC. Besides, considering the possible iatrogenic damage to the surface of the eye by surgical excision, the treatment of periocular BCCs is recommended to be performed by or in the presence of an oculoplastic surgeon.
Acknowledgments
Funding: This study was supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne, Germany (No. 2680148101) and the State Scholarship Fund from China Scholarship Council, China (No. 201708080141).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Eye Science for the series “Eyelid Surgery”. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/aes-20-100
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes-20-100). The series “Eyelid Surgery” was commissioned by the editorial office without any funding or sponsorship. LMH served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Eye Science from Dec 2019 to Nov 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol 2015;151:1081-6. [Crossref] [PubMed]
- Heindl LM. Periocular basal cell carcinoma. Ophthalmologe 2020;117:93-4. [Crossref] [PubMed]
- Oliphant H, Oliphant T, Clarke L, et al. Access to intraoperative tumour margin control: a survey of the British Oculoplastic Surgery Society. Eye (Lond) 2020;34:1679-84. [Crossref] [PubMed]
- Lang BM, Balermpas P, Bauer A, et al. S2k guidelines for cutaneous basal cell carcinoma - part 1: epidemiology, genetics and diagnosis. J Dtsch Dermatol Ges 2019;17:94-103. [Crossref] [PubMed]
- Kakkassery V, Loeffler KU, Sand M, et al. Current diagnostics and therapy recommendations for ocular basal cell carcinoma. Ophthalmologe 2017;114:224-36. [Crossref] [PubMed]
- Kakkassery V, Heindl LM. SOP - Standarized procedures in diagnostics and therapies of periocular basal cell carcinoma. Klin Monbl Augenheilkd 2017. [Epub ahead of print]. doi:
10.1055/s-0043-120086 .10.1055/s-0043-120086 - Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008;159:35-48. [Crossref] [PubMed]
- Furdova A, Lukacko P. Periocular basal cell carcinoma predictors for recurrence and infiltration of the orbit. J Craniofac Surg 2017;28:e84-7. [Crossref] [PubMed]
- Allali J, D'Hermies F, Renard G. Basal cell carcinomas of the eyelids. Ophthalmologica 2005;219:57-71. [Crossref] [PubMed]
- Conway RM, Themel S, Holbach LM. Surgery for primary basal cell carcinoma including the eyelid margins with intraoperative frozen section control: comparative interventional study with a minimum clinical follow up of 5 years. Br J Ophthalmol 2004;88:236-8. [Crossref] [PubMed]
- Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res 2005;571:91-106. [Crossref] [PubMed]
- Jayaraman SS, Rayhan DJ, Hazany S, et al. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol 2014;134:213-20. [Crossref] [PubMed]
- Boeckmann L, Martens MC, Kakkassery V, et al. Molecular genetic investigations as the basis for targeted treatment of basal cell carcinoma of the eye. Ophthalmologe 2020;117:106-12. [Crossref] [PubMed]
- Martens MC, Seebode C, Lehmann J, et al. Photocarcinogenesis and skin cancer prevention strategies: an update. Anticancer Res 2018;38:1153-58. [PubMed]
- Seebode C, Lehmann J, Emmert S. Photocarcinogenesis and skin cancer prevention strategies. Anticancer Res 2016;36:1371-8. [PubMed]
- Vink AA, Roza L. Biological consequences of cyclobutane pyrimidine dimers. J Photochem Photobiol B 2001;65:101-4. [Crossref] [PubMed]
- Lehmann J, Schubert S, Emmert S. Xeroderma pigmentosum: diagnostic procedures, interdisciplinary patient care, and novel therapeutic approaches. J Dtsch Dermatol Ges 2014;12:867-72. [Crossref] [PubMed]
- Nikolouzakis TK, Falzone L, Lasithiotakis K, et al. Current and future trends in molecular biomarkers for diagnostic, prognostic, and predictive purposes in non-melanoma skin cancer. J Clin Med 2020;9:2868. [Crossref] [PubMed]
- Bonilla X, Parmentier L, King B, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet 2016;48:398-406. [Crossref] [PubMed]
- Emmert S, Schon MP, Haenssle HA. Molecular biology of basal and squamous cell carcinomas. Adv Exp Med Biol 2014;810:234-52. [Crossref] [PubMed]
- Athar M, Li C, Kim AL, et al. Sonic hedgehog signaling in Basal cell nevus syndrome. Cancer Res 2014;74:4967-75. [Crossref] [PubMed]
- Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015;163:811-28. [Crossref] [PubMed]
- Heitzer E, Bambach I, Dandachi N, et al. PTCH promoter methylation at low level in sporadic basal cell carcinoma analysed by three different approaches. Exp Dermatol 2010;19:926-8. [Crossref] [PubMed]
- Goldberg M, Rummelt C, Laerm A, et al. Epigenetic silencing contributes to frequent loss of the fragile histidine triad tumour suppressor in basal cell carcinomas. Br J Dermatol 2006;155:1154-8. [Crossref] [PubMed]
- Darr OA, Colacino JA, Tang AL, et al. Epigenetic alterations in metastatic cutaneous carcinoma. Head Neck 2015;37:994-1001. [Crossref] [PubMed]
- Rao RC, Chan MP, Andrews CA, et al. EZH2, proliferation rate, and aggressive tumor subtypes in cutaneous basal cell carcinoma. JAMA Oncol 2016;2:962-3. [Crossref] [PubMed]
- Sand M, Bechara FG, Gambichler T, et al. Next-generation sequencing of the basal cell carcinoma miRNome and a description of novel microRNA candidates under neoadjuvant vismodegib therapy: an integrative molecular and surgical case study. Ann Oncol 2016;27:332-8. [Crossref] [PubMed]
- Yi R, Poy MN, Stoffel M, et al. A skin microRNA promotes differentiation by repressing 'stemness'. Nature 2008;452:225-9. [Crossref] [PubMed]
- Sonkoly E, Lovén J, Xu N, et al. MicroRNA-203 functions as a tumor suppressor in basal cell carcinoma. Oncogenesis 2012;1:e3 [Crossref] [PubMed]
- Rippey JJ. Why classify basal cell carcinomas? Histopathology 1998;32:393-8. [Crossref] [PubMed]
- Kerr JF, Searle J. A suggested explanation for the paradoxically slow growth rate of basal-cell carcinomas that contain numerous mitotic figures. J Pathol 1972;107:41-4. [Crossref] [PubMed]
- Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol 1990;23:1118-26. [Crossref] [PubMed]
- Lang BM, Balermpas P, Bauer A, et al. S2k guidelines for cutaneous basal cell carcinoma - part 2: treatment, prevention and follow-up. J Dtsch Dermatol Ges 2019;17:214-30. [Crossref] [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. editors. TNM classification of malignant tumours. 8th ed. Oxford: Wiley Blackwell, 2017.
- O'Sullivan B, Brierley JD, D'Cruz A, et al. editors. UICC manual of clinical oncology. 9th ed. Oxford: Wiley Blackwell, 2015.
- Kopecky A, Rokohl AC, Heindl LM. Techniques for the Reconstruction of the Posterior Eyelid Lamella. Klin Monbl Augenheilkd 2018;235:1415-28. [PubMed]
- Phan K, Oh LJ, Goyal S, et al. Recurrence rates following surgical excision of periocular basal cell carcinomas: systematic review and meta-analysis. J Dermatolog Treat 2020;31:597-601. [Crossref] [PubMed]
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol 2012;67:531-50. [Crossref] [PubMed]
- Rokohl AC, Kopecky A, Guo Y, et al. Surgical resection with ophthalmoplastic reconstruction: gold standard in periocular basal cell carcinoma. Ophthalmologe 2020;117:95-105. [Crossref] [PubMed]
- Hauschild A, Breuninger H, Kaufmann R, et al. Brief S2k guidelines--Basal cell carcinoma of the skin. J Dtsch Dermatol Ges 2013;11 Suppl 3:10-5, 11-6.
- Rokohl AC, Loser H, Mor JM, et al. Young male patient with unusual space-occupying lesion of the lower eyelid. Ophthalmologe 2020;117:73-7. [Crossref] [PubMed]
- Ozgur O, Kothapudi VN, Rostami S. Lower Eyelid Reconstruction. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing, 2020.
- Mandal SK, Fleming JC, Reddy SG, et al. Total upper eyelid reconstruction with modified cutler-beard procedure using autogenous auricular cartilage. J Clin Diagn Res 2016;10:NC01-4. [Crossref] [PubMed]
- Hishmi AM, Koch KR, Matthaei M, et al. Modified Hughes procedure for reconstruction of large full-thickness lower eyelid defects following tumor resection. Eur J Med Res 2016;21:27. [Crossref] [PubMed]
- Rohrich RJ, Zbar RI. The evolution of the Hughes tarsoconjunctival flap for the lower eyelid reconstruction. Plast Reconstr Surg 1999;104:518-22; quiz 523; discussion 524-6.
- Leibovitch I, Selva D. Modified Hughes flap: division at 7 days. Ophthalmology 2004;111:2164-7. [Crossref] [PubMed]
- McNab AA, Martin P, Benger R, et al. A prospective randomized study comparing division of the pedicle of modified hughes flaps at two or four weeks. Ophthalmic Plast Reconstr Surg 2001;17:317-9. [Crossref] [PubMed]
- Emesz M, Krall E, Nischler C, et al. Hughes' operation and combined procedures. Ophthalmologe 2014;111:448-53. [Crossref] [PubMed]
- Peris K, Fargnoli MC, Garbe C, et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer 2019;118:10-34. [Crossref] [PubMed]
- Kakkassery V, Emmert S, Adamietz IA, et al. Alternative treatment options for periorbital basal cell carcinoma. Ophthalmologe 2020;117:113-23. [Crossref] [PubMed]
- Ballester-Sánchez R, Pons-Llanas O, Candela-Juan C, et al. Electronic brachytherapy for superficial and nodular basal cell carcinoma: a report of two prospective pilot trials using different doses. J Contemp Brachytherapy 2016;8:48-55. [Crossref] [PubMed]
- Delishaj D, Rembielak A, Manfredi B, et al. Non-melanoma skin cancer treated with high-dose-rate brachytherapy: a review of literature. J Contemp Brachytherapy 2016;8:533-40. [Crossref] [PubMed]
- Duinkerken CW, Lohuis P, Crijns MB, et al. Orthovoltage X-rays for postoperative treatment of resected basal cell carcinoma in the head and neck area. J Cutan Med Surg 2017;21:243-49. [Crossref] [PubMed]
- Rishi A, Hui Huang S, O'Sullivan B, et al. Outcome following radiotherapy for head and neck basal cell carcinoma with 'aggressive' features. Oral Oncol 2017;72:157-64. [Crossref] [PubMed]
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomized study. Br J Cancer 1997;76:100-6. [Crossref] [PubMed]
- Rio E, Bardet E, Ferron C, et al. Interstitial brachytherapy of periorificial skin carcinomas of the face: a retrospective study of 97 cases. Int J Radiat Oncol Biol Phys 2005;63:753-7. [Crossref] [PubMed]
- Berking C, Hauschild A, Kolbl O, et al. Basal cell carcinoma-treatments for the commonest skin cancer. Dtsch Arztebl Int 2014;111:389-95. [PubMed]
- Cho M, Gordon L, Rembielak A, et al. Utility of radiotherapy for treatment of basal cell carcinoma: a review. Br J Dermatol 2014;171:968-73. [Crossref] [PubMed]
- Taylor RE, Hatfield P, McKeown SR, et al. Radiotherapy for benign disease: current evidence, benefits and risks. Clin Oncol (R Coll Radiol) 2015;27:433-5. [Crossref] [PubMed]
- McKeown SR, Hatfield P, Prestwich RJ, et al. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br J Radiol 2015;88:20150405 [Crossref] [PubMed]
- Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol 2015;72:1021-6.e8. [Crossref] [PubMed]
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012;366:2171-9. [Crossref] [PubMed]
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med 2012;366:2180-8. [Crossref] [PubMed]
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur J Cancer 2017;86:334-48. [Crossref] [PubMed]
- Dummer R, Guminski A, Gutzmer R, et al. The 12-month analysis from Basal Cell Carcinoma Outcomes with LDE225 Treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol 2016;75:113-25.e5. [Crossref] [PubMed]
- Demirci H, Worden F, Nelson CC, et al. Efficacy of vismodegib (erivedge) for basal cell carcinoma involving the orbit and periocular area. Ophthalmic Plast Reconstr Surg 2015;31:463-6. [Crossref] [PubMed]
- Ozgur OK, Yin V, Chou E, et al. Hedgehog pathway inhibition for locally advanced periocular basal cell carcinoma and basal cell nevus syndrome. Am J Ophthalmol 2015;160:220-7.e2. [Crossref] [PubMed]
- Lauterbach B, Kakkassery V, Debus D, et al. Advanced periocular basal cell carcinoma-a therapeutic challenge. Ophthalmologe 2019;116:273-7. [Crossref] [PubMed]
- Gill HS, Moscato EE, Chang AL, et al. Vismodegib for periocular and orbital basal cell carcinoma. JAMA Ophthalmol 2013;131:1591-4. [Crossref] [PubMed]
- Sand M, Bechara FG, Gambichler T, et al. Next-generation sequencing of the basal cell carcinoma miRNome and a description of novel microRNA candidates under neoadjuvant vismodegib therapy: an integrative molecular and surgical case study. Ann Oncol 2016;27:332-8. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Fischer S, Hasan Ali O, Jochum W, et al. Anti-PD-1 therapy leads to near-complete remission in a patient with metastatic basal cell carcinoma. Oncol Res Treat 2018;41:391-4. [Crossref] [PubMed]
- Lipson EJ, Lilo MT, Ogurtsova A, et al. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer 2017;5:23. [Crossref] [PubMed]
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep 2018;4:248-50. [Crossref] [PubMed]
- Falchook GS, Leidner R, Stankevich E, et al. Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810. J Immunother Cancer 2016;4:70. [Crossref] [PubMed]
- Geisse J, Caro I, Lindholm J, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J Am Acad Dermatol 2004;50:722-33. [Crossref] [PubMed]
- Bath-Hextall F, Ozolins M, Armstrong SJ, et al. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): a multicentre, non-inferiority, randomised controlled trial. Lancet Oncol 2014;15:96-105. [Crossref] [PubMed]
- Williams HC, Bath-Hextall F, Ozolins M, et al. Surgery versus 5% imiquimod for nodular and superficial basal cell carcinoma: 5-year results of the SINS randomized controlled trial. J Invest Dermatol 2017;137:614-9. [Crossref] [PubMed]
- Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol 2009;145:1431-8. [Crossref] [PubMed]
- Hall VL, Leppard BJ, McGill J, et al. Treatment of basal-cell carcinoma: comparison of radiotherapy and cryotherapy. Clin Radiol 1986;37:33-4. [Crossref] [PubMed]
- Ramsay JR, Suhrbier A, Aylward JH, et al. The sap from Euphorbia peplus is effective against human nonmelanoma skin cancers. Br J Dermatol 2011;164:633-6. [Crossref] [PubMed]
- Brinkhuizen T, Frencken KJ, Nelemans PJ, et al. The effect of topical diclofenac 3% and calcitriol 3 mug/g on superficial basal cell carcinoma (sBCC) and nodular basal cell carcinoma (nBCC): a phase II, randomized controlled trial. J Am Acad Dermatol 2016;75:126-34. [Crossref] [PubMed]
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med 2015;373:1618-26. [Crossref] [PubMed]
- Park SM, Li T, Wu S, et al. Niacin intake and risk of skin cancer in US women and men. Int J Cancer 2017;140:2023-31. [Crossref] [PubMed]
- Kadakia KC, Barton DL, Loprinzi CL, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer 2012;118:2128-37. [Crossref] [PubMed]
Cite this article as: Guo Y, Rokohl AC, Kopecky A, Heindl LM. Periocular basal cell carcinoma—current treatment concepts. Ann Eye Sci 2021;6:18.


