
Clinical characteristics, risk factors, and prognoses of fungal keratitis caused by three common fungal species in Northern China
Introduction
Fungal keratitis (FK) is one of the severe blinding infectious eye diseases worldwide, accounts for 1–45% of all infectious corneal diseases, about one million new cases are reported each year, especially in developing countries (1,2). Due to the lack of efficient and broad-spectrum antifungal drugs, the course of the disease is liable to be protracted, and the prognosis is poor. Therefore, early diagnosis and specific treatment are essential for prevention of perforation and more serious complications.
The diagnosis of FK requires laboratory etiological examination to provide diagnostic basis, including corneal smear, fungal culture, confocal microscopy, and pathological examination. The detection sensitivity of FK was approximately 86.7–97.0%, 50.0–69.7%, 71.4–94.0%, and 90.7% (3-9). In the early stage, fungal infection was identified mainly by corneal smear and confocal microscope, but it could not be identified by which fungus infected (10). It could only rely on corneal smear, which usually took 2–3 days to grow, and positive results were confirmed in 1 week and negative results in 2 weeks (11), which was too long. With an accurate diagnosis of FK, if the antifungal drugs used before the availability of susceptibility testing are not sensitive, the best treatment timing can still be missed, and the disease may be protracted.
We tried to analyze risk factors, clinical features, and prognoses of these three common species, hoping to provide a comparatively objective basis for the targeted treatment of FK associated with different pathogens ahead of fungal culture and susceptibility testing. The fungus is an opportunistic pathogen. Fusarium and Aspergillus are the most common genera related to FK, and the rate of Alternaria keeps rising in recent years in northern China (12-15).
We present the following article in accordance with the STROBE checklist (available at http://dx.doi.org/10.21037/aes-20-118).
Methods
This retrospective case series, non-controlled study was conducted in Shandong Eye Hospital, Shandong Eye Institute, China. This retrospectively study was approved by the Institutional Review Board of Shandong Eye Hospital and adhered to the tenets of the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. From January 2007 to December 2012, a total of 371 cases of FK caused by Fusarium, Aspergillus or Streptomyces were treated in Shandong Eye Hospital, of which 203 met the standard. Patients who received systemic or topical corticosteroid treatment, or had incomplete medical records and photographs of the cornea were excluded. All cases were diagnosed with clinical suspicion and one of the laboratory examinations (corneal scraping, culture, or corneal histology). Collecting the general information and risk factors, clinical characteristics, lesion area measurement, treatment, and prognosis in these cases. According to the infected fungal species, the 203 patients were divided into groups of Fusarium (151 cases), Aspergillus (35 cases), and Alternaria (17 cases). We have completed the STROBE checklist (available at http://dx.doi.org/10.21037/aes-20-118).
General information and risk factors
Gender, age, occupation, and clinical history were abstracted as risk factors from medical records. All cases were categorized into groups in every aspect. Gender: male, female. Age: ≤20 years, 21 to 40 years, 41 to 60 years, ≥60 years. Occupation: farmers, workers, students, unemployed, and others (including teachers, doctors, and white-collar workers).
Clinical history was focused on the following risk factors: corneal trauma (plant matter, grit, and foreign iron bodies), ocular surface diseases (including ocular disease, previous surgeries, and long-term antibiotic use), contact lens wearer, and no identified risk factor at presentation.
Clinical characteristics
A dry ulcer with pseudopodia, satellite lesions, mycelium moss, endothelial plaque, immune ring, or hypopyon was the main manifestation. The duration of the symptoms at the time of the presentation (from onset to a definite diagnosis) was divided into three groups: less than 1 week, 1 to 2 weeks, and more than 2 weeks. In addition, data were collected on treatment before confirmed diagnoses, including with antifungal treatment, with no antifungal therapy, and with unknown treatment. The microbial culture of bacterial infections and corneal perforation in severe cases were also evaluated.
Lesion area measurement
All suspected infectious corneal ulcers were calculated in a masked fashion. The lesion areas in cornea were measured and grouped as: less than 10 mm2, 10 to 20 mm2, and more than 20 mm2. The calculation of the ulcer areas was important. All the clinical and standard images were taken by slit lamp microscopy in the same magnification (×10). First, a standard 5 mm optical tape picture was taken by slit lamp microscopy, and the length (a mm) was measured using the ruler tool in the Adobe Photoshop (version 7.0) software. Second, the 5 (mm) and a (mm) were amused for the diameter of a circle, and the ratio scale plate was 52/a2. Third, the lasso tool was used to select the lesions along the lesion edge, and the pixel size and the size of the image of the selected parts in the histogram were recorded according to the Pixel method to calculate the lesion area (S) in image. The actual area (C2) was (S)×52/a2. This operation required three different people to calculate the average.
Treatment and prognosis
All patients were treated with antifungal medication first. Infections that could not be controlled with medications were subjected to surgical treatment. Depending on the infection depth, infected lesion excision, conjunctival flap transplantation, lamellar keratoplasty (LK), penetrating keratoplasty (PK), evisceration or enucleation was performed. The recurrence was monitored after surgery.
Statistical analysis
SPSS software version 17.0 was used for statistical analysis. The sex ratio, average age, occupation, risk factors, lesion area, as well as clinical features between different genera were analyzed. Chi-square tests were used to compare the rates of susceptible population, risk factors and clinical features. One-way analysis of variance and independent sample t-test was used to compare lesion areas. A P value of <0.05 was considered statistically significant.
Results
General information and risk factors
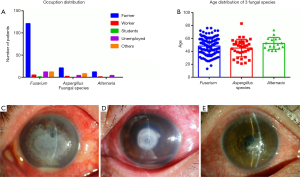
Male farmers accounted for 60.0% to 80.1%, respectively, in all patients, aged mainly from 41 to 60 years old (Figure 1A,B).
Corneal trauma was the major risk factor of FK, accounting up to 71.9% in all patients. The various genera had statistical differences in the history of trauma (P=0.015). Fusarium and Alternaria were often related to plant trauma (54.1% and 63.6%), while Aspergillus was more easily with grit (61.5%; Table 1).
Table 1
| Risk factors |
|
|
|
|---|---|---|---|
| Trauma* | 109 (72.2) | 26 (74.3) | 11 (64.7) |
| Ocular surface disease | 12 (8.0) | 2 (5.7) | 3 (17.7) |
| Contact lens wearer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 30 (19.9) | 8 (22.9) | 6 (35.3) |
| Plant trauma | 59 (54.1) | 5 (19.2) | 7 (63.6) |
| Iron | 6 (5.5) | 5 (19.2) | 0 (0.0) |
| Grit | 44 (40.4) | 16 (61.5) | 4 (36.4) |
*, compared further in classified history of trauma. Ocular eye disease: included eye ocular disease, postsurgical, long-term antibiotic use. One case in
Clinical characteristics
Main clinical features were different in three groups. The top three clinical characteristics of FK caused by Fusarium were pseudopodia (74.2%), hypopyon (39.1%), and satellite lesions (31.1%). FK caused by Aspergillus had hypopyon (51.4%), mycelium moss (45.7%), and endothelial plaque (42.9%). FK caused by Alternaria had pseudopodia (64.7%), satellite lesions (17.7%), and hypopyon (11.8%). There was significant difference in clinical features between FK caused by Aspergillus and other two groups (P=0.000, P=0.002), but not between Fusarium and Alternaria infections (P=0.446) (Table 2).
Table 2
| Clinical characteristics |
|
|
|
|---|---|---|---|
| Pseudopodia | 112 (74.2) | 15 (42.9) | 11 (64.7) |
| Satellite lesions | 47 (31.1) | 6 (17.1) | 3 (17.7) |
| Moss | 25 (16.6) | 16 (45.7) | 0 (0.0) |
| Endothelial plaque | 7 (4.6) | 15 (42.9) | 0 (0.0) |
| Immune ring | 11 (7.3) | 5 (14.3) | 1 (5.9) |
| Hypopyon | 59 (39.1) | 18 (51.4) | 2 (11.8) |
The infection duration time in three groups was mostly more than 2 weeks (from 64.7% to 74.3%). The most frequently encountered feature of FK caused by Fusarium changed with time progression (P=0.000). Pseudopodia (83.3% to 70.6%) and satellite lesions (33.3% to 44.4%) were most common in the first 2 weeks after infection. After 2 weeks, the rate of hypopyon (4.2% to 46.8%) significantly increased. The infections of Aspergillus and Alternaria did not have such significant changes (P=0.692) (Table 3, Figure 1C,D,E).
Table 3
| Clinical characteristics | Less than 1 week | 1–2 weeks, n (%) | More than 2 weeks, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|||
| Pseudopodia, n (%) | 20 (83.3) | 3 (75.0) | 1 (50.0) | 15 (83.3) | 2 (40.0) | 1 (25.0) | 77 (70.6) | 9 (34.6) | 9 (81.8) | ||
| Satellite lesions, n (%) | 8 (33.3) | 1 (25.0) | 0 (0.0) | 8 (44.4) | 1 (20.0) | 1 (25.0) | 47 (43.1) | 4 (15.4) | 2 (18.2) | ||
| Moss, n (%) | 4 (16.7) | 1 (25.0) | 0 (0.0) | 4 (22.2) | 3 (60.0) | 0 (0.0) | 22 (20.2) | 12 (46.2) | 0 (0.0) | ||
| Endothelial plaque, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.1) | 1 (20.0) | 0 (0.0) | 5 (4.6) | 14 (53.9) | 0 (0.0) | ||
| Immune ring, n (%) | 3 (12.5) | 2 (50.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 1 (25.0) | 7 (6.4) | 4 (15.4) | 0 (0.0) | ||
| Hypopyon, n (%) | 1 (4.2) | 1 (25.0) | 1 (50.0) | 6 (33.3) | 3 (60.0) | 1 (25.0) | 51 (46.8) | 14 (53.9) | 0 (0.0) | ||
| Total patient no. | 24 | 4 | 2 | 18 | 5 | 4 | 109 | 26 | 11 | ||
Lesion area
The corneal lesion area was 17.31±14.69, 13.53±10.24, and 12.0±14.0 mm2 in the Fusarium, Aspergillus, and Alternaria groups (P=0.157). 64.7% of the lesions were less than 10 mm2 in Alternaria infection, and 60.9% of the lesions were more than 10 mm2 in Fusarium infection. The lesions related to Fusarium were larger than those related to Aspergillus, especially when the area was >10 mm2, and the difference was significant (P=0.039, P=0.023). When the area was <10 mm2, the difference was mostly in Alternaria groups with other fungal groups, not between the Aspergillus and Fusarium groups (Table 4).
Table 4
| Lesion areas |
|
|
|
|||||
|---|---|---|---|---|---|---|---|---|
| Cases (%) | Lesion area (mm2) | Cases (%) | Lesion area (mm2) | Cases (%) | Lesion area (mm2) | |||
| <10 mm2 | 59 (39.1) | 4.3±2.1 | 17 (48.6) | 4.4±2.0 | 11 (64.7) | 3.7±1.3 | ||
| 10–20 mm2 | 36 (23.8) | 14.8±2.5 | 7 (20.0) | 18.7±1.2 | 3 (17.7) | 13.6±1.8 | ||
| >20 mm2 | 56 (37.1) | 35.8±11.0 | 11 (32.4) | 24.4±7.7 | 3 (17.6) | 36.5±8.3 | ||
Treatment methods and prognosis
The distribution of treatment methods for fungal infections associated with the three fungal genera is shown in Table 5. Corneal transplantation (including PK and LK) was the main treatment method for FK. The proportions of LK in the Fusarium group (31.8%), PK in the Aspergillus group (51.4%), and mere medication in the Alternaria group (17.7%) were high compared with the other two groups (Table 5).
Table 5
| Treatment methods |
|
|
|
|---|---|---|---|
| Mere medication | 13 (8.6) | 2 (5.7) | 3 (17.7) |
| Lesion excision | 8 (5.3) | 2 (5.7) | 2 (11.8) |
| Conjunctival flap | 6 (4.0) | 1 (2.9) | 1 (5.9) |
| Lamellar keratoplasty | 48 (31.8) | 7 (20.0) | 3 (17.7) |
| Penetrating keratoplasty | 61 (40.4) | 18 (51.4) | 4 (23.5) |
| Evisceration | 9 (6.0) | 1 (2.9) | 1 (5.9) |
Recurrence was found in 15 cases (9.9%) after surgical treatment in the Fusarium group, including 10 cases treated by PK, 3 cases by LK, and 2 cases by conjunctival flap transplantation. Recurrence was also detected in 2 cases (5.7%) in the Aspergillus group, both treated by PK. No recurrence was found in the Alternaria group.
Discussion
A clear understanding about features of the common FK may result in an accuracy to the diagnosis and treatment of the disease, avoid an abuse of antibiotics, and shorten the duration of symptoms.
In our study, we found that the infected patients were mainly male farmers, most aged between 41 to 60 years old, which is consistent with the data from China reported by Xie et al. and those from India by Deorukhkar et al. (12,16). This can be attributed to the eye injury becoming the first risk factor for ocular infection and the large proportion of manual labor in the agriculture of developing countries. Meanwhile, the young people wearing contact lenses have also gradually become a high-risk population of FK. In foreign reports, corneal contact lens wearing gradually become the first pathogenic factor, Candida infection even reached about 30% (17). In this current series, however, no contact lens related FK was involved, probably because the infected patients were mainly farmers and rarely wore contact lenses.
Ocular trauma accounted for 71.9% in the risk factors for FK in this study. Plant trauma was found to be the main risk factor of FK caused by Fusarium and Alternaria, while grit was the primary risk factor of Aspergillus infection. Fusarium, a plant pathogen, is widely distributed in soil, plants, and animals (18). In northern China, the commonly planted food crops are wheat, soybeans, corn, and sorghum, and Fusarium precisely is the main pathogen that causes serious disease of such crops (1). This may be the reason why FK caused by Fusarium usually had a history of plant trauma. Similarly, Alternaria also belong to filamentous fungi, which are commonly found in the environments of fruits, vegetables and field crops, can grow and reproduce in low temperature and wet environment (19). Therefore, Fusarium and Alternaria infections are mostly caused by a history of plant trauma. Aspergillus is a common saprophytic fungal pathogen in moldy grain, grain products, and other mold rot organisms. Their spores are easily spread in the air. The spores in the air and sand increase the fungal infections in the eye. Weak resistance of the eye may also increase the chances of infection, and eye diseases concerning infections with Aspergillus have been reported (20).
The clinical manifestations of different fungal infections are different. In this study, the main features of FK caused by Fusarium were pseudopodia, hypopyon, and satellite lesions. At early stage, pseudopodia, satellite lesions were predominant; with the disease progressed, hypopyon increased gradually. With the extension of the disease course, the incidence of hypopyon, mainly manifested as pseudopodia, satellite lesions, and hypopyon. The clinical characteristics of each course were different. Due to the Fusarium hyphae was usually parallel to the corneal lamellae, and easily spread to the surrounding stroma, presenting with pseudopodia and satellite lesions (21-24). Histopathology of corneal lesions by Bai et al. found thickening of Fusarium hyphae wall in Fusarium infection and a hypha-in-hypha structure (23). It regarded as a protective device for the survival of F. solani to evade the host’s immune system (21). Transmission electron microscopy revealed the dissolution of collagen fibers around Fusarium spores, suggesting that Fusarium may produce enzymes that degrade collagen fibers (23). The corneal tissue may become necrotic when a large number of hyphae proliferated together with inflammatory cell infiltration. For these interact factors, the hyphae invaded into the deep cornea stroma, caused anterior chamber reaction, and developed hypopyon with the progression of the disease.
In this series, FK caused by Aspergillus was featured with moss, endothelial plaque, and pseudopodia, and there were no significant differences between different courses. The growth pattern of Aspergillus in the cornea is mainly vertical and obtuse. Bai et al. also found that Fusarium can be surrounded by neutrophils and phagocytic, while Aspergillus has not been found to be phagocytic, suggesting that Aspergillus has a different survival pattern from Fusarium (23). Transmission electron microscopy showed that collagen fibers dissolved around the mycelia and spores of Aspergillus, suggesting that Aspergillus also produces enzymes that degrade collagen fibers (23). The expression of matrix metalloproteinase (MMPs) appears in the corneal tissue of FK at an early stage, and the expression of MMP-9 is positively correlated with the degree of clinical infection. A large amount of MMP-9 is released by neutrophils, which causes the degradation and destruction of corneal epithelial basement membrane and Matrix collagen, and promotes the spread of infection in depth (25). Expression of MMP-9 significantly more than the Fusarium, serious degradation of collagen corneal damage, hyphae to early through corneal lamellar are vertical or oblique growth way (25), so the necrosis and destruction of the corneal stroma is more severe, making it more easily for the hyphae to invade into the deep stroma of the cornea with serious clinical features. Hypopyon and endothelial plaque usually occurred at the early stage of Aspergillus infection.
The primary manifestations of FK caused by Alternaria were pseudopodia and satellite lesions, but rarely accompanied with hypopyon in our study. Because of the low invasiveness to the cornea, Alternaria usually causes a superficial ulcer, with no progression in a long period of time (26). We detected smaller lesions and more stable clinical manifestations in the Alternaria infection than the Fusarium infection. It may be associated with the parallel hyphal growth (27) and low invasiveness of Alternaria.
There was no significant difference in treatment methods among the infections with various genuses. PK was most frequently performed, followed by LK, which may be attributed to that most patients had a long course before referral to our hospital. The infection became very severe, and surgical interventions were required for these patients who were unresponsive to antifungal medication. For the stubborn FK, the recurrence could not be avoided. In this study, the rate of postoperative recurrence was about 9.9% in Fusarium infection, 66.7% after PK, 5.7% in Aspergillus infection, and all in LK. The reason may be related to the growth pattern of different fungal strains. The parallel growth of Fusarium hyphae in the cornea makes it not easy to excise all fungal hyphae under a microscope during the surgery (28). For the Aspergillus, the hyphae grow perpendicular to the stromal collagen, so the infection is liable to penetrate into the deep stroma. Recurrence may occur from the recipient after LK in the Aspergillus group (28,29). Therefore, selection of an appropriate surgical approach according to the fungal genus may improve the success rate of surgery.
The limitation of this study is that most patients were from other hospital, and have already received antibiotic treatment when we visited them. This may affect the clinical characters. Also, although empirical treatments have advantage before the culture results is available, it cannot substitute for microbiological testing during the initial clinical encounter. The antifungal drug treatment should adjust to tailored treatment when susceptibility testing is available. AI-assisted diagnosis has been proposed and studied for many years (30,31). Although the application of this technology is still in the initial stage, due to its unique advantages. The image of anterior segment combined with artificial intelligence will have an optimistic clinical effect on the diagnosis of FK.
Conclusions
Risk factors and clinical characteristics of FK caused by different genuses were varied. Fusarium and Alternaria were usually related with plant trauma, while Aspergillus was more easily with grits. The Fusarium and Alternaria infections usually presented with pseudopodia and satellite lesions, but the Alternaria infection usually had sensitive response to drug treatment. Aspergillus often had hypopyon, mycelium moss, and endothelial plaque. Adequate understanding of the clinical characteristics of FK caused by different genuses can help for early diagnosis and target medication before the drug sensitive results are available.
Acknowledgments
The authors thank Ping Lin, Shandong Eye Institute, Qingdao, China for her linguistic assistance.
Funding: This study was supported by National Natural Science Foundation of China (82070923, 81870639), Key Project of National Natural Science Foundation of China (81530027), Taishan Scholar Program (20150215, 201812150), and the Innovation Project of Shandong Academy of Medical Sciences, Academic Promotion Programme of Shandong First Medical University (2019RC009).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Xiulan Zhang) for the series “Ophthalmology Clinical Research” published in Annals of Eye Science. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE checklist. Available at http://dx.doi.org/10.21037/aes-20-118
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aes-20-118
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes-20-118). The series “Ophthalmology Clinical Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shandong Eye Hospital and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shah A, Sachdev A, Coggon D, et al. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol 2011;95:762-7. [Crossref] [PubMed]
- Maharana PK, Sharma N, Nagpal R, et al. Recent advances in diagnosis and management of Mycotic Keratitis. Indian J Ophthalmol 2016;64:346-57. [Crossref] [PubMed]
- Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol 2001;85:1070-4. [Crossref] [PubMed]
- Shi W, Li S, Liu M, et al. Antifungal chemotherapy for fungal keratitis guided by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol 2008;246:581-6. [Crossref] [PubMed]
- Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol 2009;57:273-9. [Crossref] [PubMed]
- Kheirkhah A, Syed ZA, Satitpitakul V, et al. Sensitivity and Specificity of Laser-Scanning In Vivo Confocal Microscopy for Filamentous Fungal Keratitis: Role of Observer Experience. Am J Ophthalmol 2017;179:81-9. [Crossref] [PubMed]
- Yue X, Wang A, Wang H, et al. Evaluation of a new fluorescent reagent, fluorescent brightener 85, for the diagnosis of suspected onychomycosis compared with potassium hydroxide. Mycoses 2018;61:279-82. [Crossref] [PubMed]
- Zhang H, Dou X, Yang K, et al. Feasibility of confocal laser scanning microscopy with slit-lamp microscope in the diagnosis of filamentous fungal corneal infection with culture negative patients. Chin J Exp Ophthalmol 2018;36:119-23.
- Zhang Y, Wang Z, Deng S, et al. Diagnostic value of fungal fluorescence staining on corneal scrapings for fungal keratitis. Zhonghua Yan Ke Za Zhi 2019;55:601-8. [PubMed]
- Tabatabaei SA, Soleimani M, Tabatabaei SM, et al. The use of in vivo confocal microscopy to track treatment success in fungal keratitis and to differentiate between Fusarium and Aspergillus keratitis. Int Ophthalmol 2020;40:483-91. [Crossref] [PubMed]
- Maldonado C, Cauli O, Rodríguez-Arias M, et al. Memantine presents different effects from MK-801 in motivational and physical signs of morphine withdrawal. Behav Brain Res 2003;144:25-35. [Crossref] [PubMed]
- Xie L, Zhong W, Shi W, et al. Spectrum of fungal keratitis in north China. Ophthalmology 2006;113:1943-8. [Crossref] [PubMed]
- Bai L, Xia J. Retrospective Study of Fungal Keratitis in 412 Patients. Chin J Optom Ophthalmol Vis Sci 2019;21:865-70.
- Chew R, Woods ML. Epidemiology of fungal keratitis in Queensland, Australia. Clin Exp Ophthalmol 2019;47:26-32. [Crossref] [PubMed]
- Manikandan P, Abdel-Hadi A, Randhir BSY, et al. Fungal Keratitis: Epidemiology, Rapid Detection, and Antifungal Susceptibilities of Fusarium and Aspergillus Isolates from Corneal Scrapings. Biomed Res Int 2019;2019:6395840 [Crossref] [PubMed]
- Deorukhkar S, Katiyar R, Saini S. Epidemiological features and laboratory results of bacterial and fungal keratitis: a five-year study at a rural tertiary-care hospital in western Maharashtra, India. Singapore Med J 2012;53:264-7. [PubMed]
- Ong HS, Fung SS, Macleod D, et al. Altered Patterns of Fungal Keratitis at a London Ophthalmic Referral Hospital: An Eight-Year Retrospective Observational Study. Am J Ophthalmol 2016;168:227-36. [Crossref] [PubMed]
- Dóczi I, Gyetvai T, Kredics L, et al. Involvement of Fusarium spp. in fungal keratitis. Clin Microbiol Infect 2004;10:773-6. [Crossref] [PubMed]
- Man Y, Liang G, Li A, et al. Research progress of detecting methods for Alternaria toxins. J Food Saf Food Qual 2016;7:453-8.
- Weiss K, Ardjomand N, El-Shabrawi Y. Mycotic infections of the eye. Wien Med Wochenschr 2007;157:517-21. [Crossref] [PubMed]
- Kiryu H, Yoshida S, Suenaga Y, et al. Invasion and survival of Fusarium solani in the dexamethasone-treated cornea of rabbits. J Med Vet Mycol 1991;29:395-406. [Crossref] [PubMed]
- Wu TG, Keasler VV, Mitchell BM, et al. Immunosuppression affects the severity of experimental Fusarium solani keratitis. J Infect Dis 2004;190:192-8. [Crossref] [PubMed]
- Bai H, Jin M, Zhao G, et al. Histopathological features of Fusarium and Aspergillus corneal ulcer. Chin J Ophthalmol 2004;40:341-3.
- Dong X, Shi W, Zeng Q, et al. Roles of adherence and matrix metalloproteinases in growth patterns of fungal pathogens in cornea. Curr Eye Res 2005;30:613-20. [Crossref] [PubMed]
- Zeng Q, Dong X, Shi W, et al. Role of fungal spore adhesion and matrix metalloproteinases in corneal fungal infection. Chin J Ophthalmol 2004;40:774-6.
- Garg P, Vemuganti GK, Chatarjee S, et al. Pigmented plaque presentation of dematiaceous fungal keratitis: a clinicopathologic correlation. Cornea 2004;23:571-6. [Crossref] [PubMed]
- Liu X, Wu S, Rong J, et al. Clinical characteristics and outcomes of keratitis caused by different fungal species. Recent Adv Ophthalmol 2016;36:250-4.
- Shi W, Wang T, Xie L, et al. Risk factors, clinical features, and outcomes of recurrent fungal keratitis after corneal transplantation. Ophthalmology 2010;117:890-6. [Crossref] [PubMed]
- Xie L, Hu J, Shi W. Treatment failure after lamellar keratoplasty for fungal keratitis. Ophthalmology 2008;115:33-6. [Crossref] [PubMed]
- Scerri M, Grech V. Artificial intelligence in medicine. Early Hum Dev 2020;145:105017 [Crossref] [PubMed]
- Liu Z, Cao Y, Li Y, et al. Automatic diagnosis of fungal keratitis using data augmentation and image fusion with deep convolutional neural network. Comput Methods Programs Biomed 2020;187:105019 [Crossref] [PubMed]
Cite this article as: Wang X, Li S, Jia Y, Qi X, Shi W, Gao H. Clinical characteristics, risk factors, and prognoses of fungal keratitis caused by three common fungal species in Northern China. Ann Eye Sci 2020;5:34.


