
Advances in the diagnosis and management of acute retinal necrosis
Introduction
Acute retinal necrosis (ARN) is a rare viral uveitis syndrome that results in devastating visual consequences, often even if properly diagnosed and treated. It was first described in Japan in 1971 by Urayama et al. as a syndrome characterized by unilateral panuveitis, vitritis, acute necrotizing retinitis, retinal arteritis, and rhegmatogenous retinal detachment, but the first evidence of its herpetic etiology was reported by Culbertson et al. in 1982 (1,2). Since then, multiple viruses in the herpesvirus family have been found to be causative agents, with varicella zoster virus (VZV) identified as the most common etiology, followed by herpes-simplex virus 1 and 2 (HSV-1, HSV-2), cytomegalovirus (CMV), and Epstein-Barr virus (3,4). Clinical treatment has been guided primarily by retrospective case series, case reports, and expert opinion; nonetheless, significant advances have been made to establish prompt diagnosis and treatment of this potentially destructive panuveitis syndrome.
Epidemiology
The exact incidence of ARN is unknown, but two surveys in the UK estimated an incidence of 1 case per 1.6 to 2.0 million population per year (5,6). There are 50,000 new and recurring cases of ocular manifestations of HSV in the U.S. each year, and the disease burden will likely increase due to the aging population and increased number of immunosuppressed individuals (7). ARN has no identified predilection with regard to race or gender, but a genetic association has been shown in Caucasian patients with the HLA-DQw7 antigen and HLA-Bw62 phenotype, suggesting a possible immune predisposition for developing ARN (8). Furthermore, ARN most commonly affects otherwise healthy adults, contrasting progressive outer retinal necrosis (PORN), another subtype of herpetic retinopathy that affects immunocompromised patients. A temporal pattern of incidence of ARN has been observed in one report, with cold-weather seasons preceding the highest rates of infection. This is consistent with patterns of seasonal variability observed in other types of infections associated with the Herpesviridae family (9).
Diagnosis
In 1994, the American Uveitis Society established standard diagnostic criteria for ARN based on clinical presentation and disease course, including: (I) 1 or more foci of retinal necrosis with discrete borders located in the peripheral retina; (II) rapid progression in the absence of antiviral therapy; (III) circumferential spread; (IV) evidence of occlusive vasculopathy with arterial involvement; (V) prominent inflammatory reaction in vitreous and anterior chamber. The differential diagnosis for patients presenting with similar symptoms includes infectious and non-infectious entities, such as toxoplasma chorioretinitis, CMV retinitis, syphilis, Behcet’s disease, lupus vasculitis, and sarcoidosis (10). Primary or secondary vitreoretinal lymphoma may also present with features mimicking ARN (11).
Since the diagnostic criteria was established in 1994, advances in polymerase chain reaction (PCR) techniques and widespread availability of the test have made it possible to accurately identify the causative virus. While laboratory data has not been included in the diagnostic criteria for ARN, PCR testing of anterior chamber or vitreous fluid has become increasingly valuable in diagnosis and is warranted in all patients where testing is available. These tests are highly sensitive with a virus detection rate in 79–100% of cases with suspected ARN (12). Given the high sensitivity of PCR diagnostics, a negative test should prompt the clinician to consider alternative diagnoses or conversely, consider retesting the aqueous humor sample if these is a suspicion for a false negative result. A number of studies have compared the detection rates of aqueous and vitreous fluid samples with no clear difference in the ocular fluid assessed (12,13).
Quantitative PCR testing can not only identify the specific virus to assist in treatment but may also assist the clinician to follow intraocular DNA levels during patient treatment or if the disease appears recalcitrant to systemic and intravitreal therapy. Viral load monitoring can provide evidence for prognosis and resistance to antiviral treatment. High DNA copy number has been associated with worse visual acuity, more extensive retinitis, and development of retinal detachment (14,15); however, a retrospective case series by Von Hofsten et al. found no correlation between viral load and visual outcome (16) and Sato, et al. found no change in viral load in patients after IV acyclovir treatment (13). Another study of viral load kinetics of ARN patients found a 3-phase change in viral load through the disease course, and treatment resistance was associated with a prolonged initial plateau period of viral load (17). Further evidence is needed regarding the value of viral load for monitoring disease.
Clinical features and outcomes
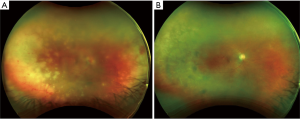
ARN presents with a clinical presentation of acute panuveitis with findings that may include anterior uveitis, scleritis, vitritis, occlusive vasculitis, necrotizing retinitis, and optic disc edema (Figure 1A). Visual outcomes may be poor in some patients, with one report describing visual acuity (VA) worse than 20/200 six months after onset of symptoms in half of their patients (5). A number of patient-specific and clinical prognostic factors have been studied for correlation with visual outcomes. Poorer initial visual acuity, delay in treatment, increased total area of retinitis, and zone I involvement of the retina (one disc diameter surrounding the disc and 2 disc diameters around the fovea) may portend a poorer visual prognosis and higher incidence of retinal detachment (18-20). The rate of retinal detachment (RD) varies in the literature and has ranged from 20–73% of treated eyes but is the most common cause of vision loss. Other possible vision-compromising complications include optic atrophy, cystoid macular edema, retinal atrophy, macular hole, and epiretinal membrane formation (21). The morbidity of ARN increases with central nervous system, such as encephalitis or meningitis, and contralateral eye involvement (12). Bilateral disease, either simultaneous or sequential, occurs in up to 70% of untreated patients and up to 90% of immunocompromised patients, and contralateral disease can occur from a few months to years later (22-24). In a study of 54 patients by Palay et al., 31 patients were treated with acyclovir while 23 were not. Twenty-seven (87%) patients treated with acyclovir remained disease-free in the fellow eye, whereas only seven (30%) patients not receiving acyclovir remained disease-free at a median of 11 months of follow-up (23).
The course of ARN is often complicated by secondary RD. ARN has the highest incidence of RD compared to all other causes of uveitis, and patients with VZV have been reported to have a 2.5 times greater risk of RD than those with HSV (25). A retrospective study by Butler et al. showed that RD was associated with the greatest risk of vision loss, followed by retinitis of more than 25% of the retina (26). A variety of surgical approaches have been reported for RDs in the setting of ARN, which are complicated due to diffuse retinal necrosis with multiple retinal breaks, often posteriorly located. While no difference has been observed when comparing outcomes between PPV with silicone-oil tamponade versus scleral buckling as repair methods for RD, visual acuity may also be limited by optic neuropathy, macular ischemia, and macula-off status at the time of the RD (27). Visual prognosis after primary repair of RD may remain poor despite surgical intervention due to high recurrence of RD. In one study, even after surgical repair, 46.2% of eyes developed recurrent RD, and the same number developed severe vision loss (28). Dave, et al. showed that rhegmatogenous RD occurring later in the disease course, systemic corticosteroid use, and oral valacyclovir were associated with favorable visual outcomes after surgical RD repair with pars plana vitrectomy (PPV) and silicone oil tamponade (29).
Treatment
Combination systemic and intravitreal antiviral therapy have become the mainstays of treatment of ARN with adjunctive therapies including corticosteroids for severe inflammation that may accompany ARN (Figure 1B). Laser photocoagulation has been proposed to prevent retinal detachment. Pars plana vitrectomy has also been described to prevent retinal detachment, obtain a vitreous specimen for diagnostic purposes, and for treatment of complications of ARN including RD which may occur in a high percentage of ARN patients, particularly eyes with widespread retinitis.
Medical therapy
Despite established etiological and clinical features, the therapeutic approach for ARN is not uniform, with variations in the timing of intervention and surgical management strategies that have evolved over time. The most common initial treatment was IV acyclovir after studies showed improved outcomes, including regression of retinal lesions and decreased optic nerve involvement. Systemic antiviral therapy also appeared to decrease incidence of contralateral eye involvement in these studies. Palay et al. reported that in 54 patients with unilateral ARN, 27 (87%) had fellow eyes that remained disease disease-free when treated with acyclovir, whereas only 7 (30%) of fellow eyes remain disease-free when acyclovir was not administered (23,30). The advent of new oral antivirals with greater bioavailability than oral acyclovir has resulted in oral therapy being used more frequently as initial therapy with the benefit of outpatient management rather than inpatient IV acyclovir. Studies comparing IV acyclovir and oral valacyclovir concluded that while IV acyclovir reaches a higher maximal concentration and faster time to peak concentration, oral valacyclovir still reaches inhibitory ranges in the vitreous and is demonstrates comparable efficacy as a viral DNA polymerase inhibitor with no difference on final VA, RD, or time to regression of retinitis (31,32). In addition, a cost analysis of oral valacyclovir for 10 days versus a 7 day hospital admission and IV acyclovir course for initial therapy further justifies an oral treatment as an acceptable and often preferable alternative to inpatient IV therapy (12).
In addition to systemic therapy, intravitreal therapy (IVT) with foscarnet and ganciclovir provide direct and immediate treatment for active infection. Comparative studies assessing the role of intravitreal foscarnet showed benefit in using combination systemic and intravitreal therapy compared to systemic therapy alone. Intravitreal therapy cannot be used alone due to the risk of contralateral involvement without systemic therapy. Flaxel et al. reported reduced vison loss and incidence of RD, and Wong et al. showed a decrease in risk of RD by 67% in patients with systemic antiviral and intravitreal foscarnet (21,33,34). A recent study showed that increased number and prolonged course of intravitreal injections, with weekly injections until an undetectable aqueous humor viral load is reached, improves prognosis by decreasing risk of RD and improving VA (17).
For patients with refractory disease despite standard first-line therapy, escalation of therapy should be considered. There are several case reports of patients who responded well to intravenous foscarnet after initial treatment failure (35-37). In addition, the use of intravitreal ganciclovir has been shown to be synergistic with foscarnet and improve outcomes in patients with refractory disease (38,39). The use of biologics, while not yet a mainstay of treatment, is starting to be explored. Bauer et al. studied an HSV glycoprotein-specific antibody as an option for acyclovir-resistant ocular disease that ultimately protected mouse models from developing HSV-1 induced ARN (40,41).
Use of corticosteroids and other adjunctive therapies
Given that ARN may present with a severe inflammatory response with clinical findings that include anterior uveitis, vitritis, and necrotizing vasculitis, immunomodulatory agents are a strong consideration after or concurrent with antiviral therapy. Topical and systemic corticosteroids can be considered in patients with severe inflammation; however, caution with corticosteroid is advisable as early initiation of corticosteroid treatment may potentiate viral replication and induce rapid progression of retinitis. Extensive retinitis with vascular occlusion may occur in patients with ARN treated with intravenous corticosteroid prior to initiation of antiviral therapy (42,43). Most patients receive topical steroids during the initial treatment period, and oral corticosteroids can be added 24–48 hours after the start of antiviral therapy in certain cases to minimize vitritis and the risk of retinal detachment (44). One case report of a patient treated with antiviral and simultaneous dexamethasone implant showed the success of treating intraocular inflammation with targeted steroids (39). Majumder, et al. reported two cases of dexamethasone implants in patients with CME following resolution of uveitis who had resolution of edema with no recurrence of retinitis (45). Despite success with corticosteroid therapy, the risk of disease exacerbation warrants its judicious use and close follow-up (46).
Vascular occlusion and retinal ischemia are common features of ARN. While there is not yet evidence for the use of anticoagulation with heparin and warfarin, platelet hyperaggregation has successfully been treated with corticosteroids and aspirin (47).
Laser photocoagulation, vitrectomy and surgical considerations
Because of the high incidence of retinal tears and retinal detachments that develop secondary to retinal necrosis, prophylactic measures prior to retinal detachment development, including laser photocoagulation and PPV have been utilized.
Prophylactic laser photocoagulation creates strong chorioretinal adhesions posterior to the area of involved retina to prevent RD. Several reports have shown statistically significant decrease of RD in patients with laser prophylaxis; however, selection bias limits the interpretation of many of these results as patients who receive laser are more likely to have milder disease, less vitritis and lesions more amenable to laser photocoagulation barricade (5,18,20,48,49). Other studies report little to no benefit for patients (22,50). Risseeuw et al. (51) showed that out of 63 patients with ARN, those who underwent prophylactic laser had a higher rate of RD (45.5%) compared to no prophylactic treatment (26.7%) or pars plana vitrectomy (PPV) (14.3%). As a result, there is inconclusive evidence for the use of prophylactic laser in preventing RD in ARN patients.
PPV allows for the removal of inflammatory mediators and vitreous traction and has been paired with silicone oil tamponade for subsequent RD prevention. Studies show variable outcomes of early PPV and studies have differed with respect to baseline patient characteristics and variable follow-up. One study showed reduction in RD with PPV, but there was no difference in final VA compared to patients who did not undergo early PPV; however, Luo, et al. found both reduced RD and better final VA in the PPV group (52-54). Ishida et al. and Liu et al. found no difference in recurrent RD or final VA (55,56). Even though Risseeuw et al. observed a reduction in risk of RRD with PPV compared to laser photocoagulation, the role of PPV in preventing RD or improving visual prognosis is still unclear (51).
Even with appropriate therapy, RD is known to occur in a high proportion of ARN cases. Preoperative evaluation includes an assessment of whether the infection remains active or inactive, as this may affect the dosing of intravitreal antivirals (i.e. dosage of foscarnet or ganciclovir in an oil-filled eye). Our preference is to delay surgery until the infectious process has demonstrated improvement or resolved where possible. If the RD is localized and macula-sparing, RD repair will be undertaken with concomitant administration of an intravitreal antiviral agent at the time of silicone instillation. Surgical therapy consists of pars plana vitrectomy, endolaser, and silicone oil tamponade is generally favored to long-acting gas therapy because of the high rate of proliferative vitreoretinopathy development and risk of recurrent RD. In one series, of 15 eyes that developed RD, 13 underwent surgical repair and six patients (46%) developed RD recurrence (28). Scleral buckling is a consideration in some patients; however, the posterior extent of retinitis, clock-hours of RD, and amount of retina that needs to be supported are important considerations in whether a scleral buckle may be helpful (e.g., a patient with 12 clock-hours of RD would not derive the same benefit as a patient with an inferior, macula-on RD with a tractional component).
Role of long-term prophylactic antiviral therapy
After resolution of active ARN, some authors have suggested long-term, or even lifelong use of oral valacyclovir for the prevention of contralateral involvement. Limited evidence is available on the benefit of long-term antiviral therapy for herpetic retinitis although one early study on IV acyclovir showed a reduction in fellow eye involvement from 70% without acyclovir to 13% with acyclovir (23). The Herpetic Eye Disease Study Group previously evaluated oral acyclovir for the prevention of epithelial and stromal keratitis for patients with prior HSV eye disease and found that the cumulative probability of ocular HSV recurrence during a 1-year acyclovir treatment period was 19% in patients receiving acyclovir and 32% in patients receiving placebo (57). While this difference has not been definitive shown in ARN patients, the potential blinding complications of ARN if it occurs in the contralateral, initially unaffected eye can be profound. Dosing for long-term valacyclovir use varies between 500 mg to 1 g twice daily, but multiple factors are considered including the patient’s visual acuity in the affected eye (i.e., whether the patient is functionally monocular), the patient’s kidney function and any risk of exacerbation of kidney disease that would require renal dosing, and other HSV comorbidities (e.g., history of genital herpes, HSV encephalitis, oral ulcers). These decisions are made on a patient-by-patient basis taking into consideration the overall health of the patient, risk of medication, and risk to the fellow eye.
Conclusions
ARN is a rare infectious panuveitis syndrome with potentially, rapidly progressive, vision-threatening complications that requires prompt diagnosis, appropriate therapy, and close follow-up to ensure the best possible visual outcomes. Therapy should be started immediately in patients with suspected ARN. PCR diagnostics have greatly improved our ability to quickly and accurately identify herpetic etiology of ARN and initiate prompt antiviral therapy, and viral load detection may assist in determining prognosis and evaluating a patient’s response to treatment. A number of systemic and local therapies are available for treatment, but current evidence suggests systemic (IV or oral) antiviral with adjunctive intravitreal foscarnet as first-line initial therapy to immediately limit the extent of disease, reduce the risk of severe vision loss and incidence of RD.
While intravitreal therapeutics may control the disease locally, additional studies may refine our protocols related to the frequency and course of antiviral therapies. In addition, the combination of foscarnet and ganciclovir have been successful for cases of severe or refractory disease. The use of topical and systemic corticosteroid as adjunctive therapy improves inflammation. However, more evidence is needed to determine the timing of systemic corticosteroid therapy, as blinding complications of ARN have been reported with parenterally and locally administered corticosteroid when patients are not given concomitant antiviral therapy. The management of ARN also includes an understanding of the timing and approach to surgical repair of RD, as well as appropriate patient counseling regarding the prognosis and need for surgery in some situations. Long-term maintenance therapy with oral therapy is a strong consideration to prevent disease recurrence or contralateral involvement, but should account for the functional status of the affected eye, kidney disease, and potential risk of vision loss if the patient is functionally monocular. While significant advances have been made to diagnose and manage ARN, further studies are needed to refine disease protocols and improve outcomes for this challenging infectious uveitis syndrome.
Acknowledgments
Funding: This project was supported by the National Eye Institute/National Institutes of Health core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine), National Eye Institute of the National Institutes of Health under award RO1 EY029594 (SY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was also supported an unrestricted departmental grant from Research to Prevent Blindness, Inc. to the Emory Eye Center, Emory University School of Medicine and Association for Research in Vision and Ophthalmology Mallinckrodt Young Investigator Award (SY).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Steven Yeh) for the series “Innovations in the Diagnosis and Management of Uveitis” published in Annals of Eye Science. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes-2019-dmu-09). The series “Innovations in the Diagnosis and Management of Uveitis” was commissioned by the editorial office without any funding or sponsorship. SY served as the unpaid Guest Editor of the series. SY reports other from Clearside Biomedical, other from Santen, Inc., other from Bausch and Lomb, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Culbertson WW, Blumenkranz MS, Haines H, et al. The acute retinal necrosis syndrome. Part 2: Histopathology and etiology. Ophthalmology 1982;89:1317-25. [Crossref] [PubMed]
- Urayama A, Yamada N, Sasaki T. Unilateral acute uveitis with periarteritis and detachment. Jpn J Clin Ophthalmol 1971;25:607-19.
- Silverstein BE, Conrad D, Margolis TP, et al. Cytomegalovirus-associated acute retinal necrosis syndrome. Am J Ophthalmol 1997;123:257-8. [Crossref] [PubMed]
- Walters G, James TE. Viral causes of the acute retinal necrosis syndrome. Curr Opin Ophthalmol 2001;12:191-5. [Crossref] [PubMed]
- Cochrane TF, Silvestri G, McDowell C, et al. Acute retinal necrosis in the United Kingdom: results of a prospective surveillance study. Eye (Lond) 2012;26:370-7; quiz 8. [Crossref] [PubMed]
- Muthiah MN, Michaelides M, Child CS, et al. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol 2007;91:1452-5. [Crossref] [PubMed]
- Weiner G. Demystifying the ocular herpes simplex virus: American Academy of Ophthalmology; 2013. Available online: www.aao.org/publications/eyenet/201301/feature.cfm
- Holland GN, Cornell P, Park M. An association between acute retinal necrosis syndrome and HLA-DQw7 and phenotype Bw62, DR4. Am J Ophthalmol 1989;108:370-4. [Crossref] [PubMed]
- Hedayatfar A, Khorasani MA, Behnia M, et al. Seasonality of Acute Retinal Necrosis. J Ophthalmic Vis Res 2020;15:53-8. [PubMed]
- Crosson JN, Kuthyar S, Shantha JG, et al. Toxoplasmosis chorioretinitis mimicking acute retinal necrosis associated with local corticosteroid. Int J Retina Vitreous 2020;6:21. [Crossref] [PubMed]
- Ryan ME, Shantha JG, Grossniklaus HE, et al. Secondary Vitreoretinal Lymphoma Masquerading as Acute Retinal Necrosis. Ophthalmic Surg Lasers Imaging Retina 2015;46:1048-50. [Crossref] [PubMed]
- Schoenberger SD, Kim SJ, Thorne JE, et al. Diagnosis and Treatment of Acute Retinal Necrosis. Ophthalmology 2017;124:382-92. [Crossref] [PubMed]
- Sato T, Yamamoto W, Tanaka A, et al. Viral Loads in Ocular Fluids of Acute Retinal Necrosis Eyes Infected by Varicella-Zoster Virus Treated with Intravenous Acyclovir Treatment. J Clin Med 2020;9:1204. [Crossref] [PubMed]
- Calvo CM, Khan MA, Mehta S, et al. Correlation of Clinical Outcomes with Quantitative Polymerase Chain Reaction DNA Copy Number in Patients with Acute Retinal Necrosis. Ocul Immunol Inflamm 2017;25:246-52. [Crossref] [PubMed]
- Abe T, Sato M, Tamai M. Correlation of varicella-zoster virus copies and final visual acuities of acute retinal necrosis syndrome. Graefes Arch Clin Exp Ophthalmol 1998;236:747-52. [Crossref] [PubMed]
- von Hofsten J, Bergström T, Zetterberg M. Alpha herpes virus type and viral load in intraocular fluids in patients with acute retinal necrosis. BMJ Open Ophthalmol 2019;4:e000247 [Crossref] [PubMed]
- Hafidi M, Janin-Manificat H, Denis P, et al. Acute Retinal Necrosis: Virological Features Using Quantitative Polymerase Chain Reaction, Therapeutic Management, and Clinical Outcomes. Am J Ophthalmol 2019;208:376-86. [Crossref] [PubMed]
- Kim DY, Jo J, Joe SG, et al. Clinical feature and visual prognosis of acute retinal necrosis according to the initially involved zone and extent: 10-year experience. Eur J Ophthalmol 2019;29:244-50. [Crossref] [PubMed]
- Mora P, Zola M, Favilla S, et al. Visual outcome and poor prognostic factors in acute retinal necrosis syndrome. Graefes Arch Clin Exp Ophthalmol 2020;258:1851-6. [Crossref] [PubMed]
- Meghpara B, Sulkowski G, Kesen MR, et al. Long-term follow-up of acute retinal necrosis. Retina 2010;30:795-800. [Crossref] [PubMed]
- Wong RW, Jumper JM, McDonald HR, et al. Emerging concepts in the management of acute retinal necrosis. Br J Ophthalmol 2013;97:545-52. [Crossref] [PubMed]
- Tibbetts MD, Shah CP, Young LH, et al. Treatment of Acute Retinal Necrosis. Ophthalmology 2010;117:818-24. [Crossref] [PubMed]
- Palay DA, Sternberg P Jr, Davis J, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol 1991;112:250-5. [Crossref] [PubMed]
- Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol 2005;20:155-60. [Crossref] [PubMed]
- Kunavisarut P, Srisomboon T, Patikulsila D, et al. Risk Factors for Development of Rhegmatogenous Retinal Detachment in Patients with Uveitis. Ocul Immunol Inflamm 2019;27:681-5. [Crossref] [PubMed]
- Butler NJ, Moradi A, Salek SS, et al. Acute Retinal Necrosis: Presenting Characteristics and Clinical Outcomes in a Cohort of Polymerase Chain Reaction-Positive Patients. Am J Ophthalmol 2017;179:179-89. [Crossref] [PubMed]
- Almeida DR, Chin EK, Tarantola RM, et al. Long-term outcomes in patients undergoing vitrectomy for retinal detachment due to viral retinitis. Clin Ophthalmol 2015;9:1307-14. [PubMed]
- Kopplin LJ, Thomas AS, Cramer S, et al. Long-Term Surgical Outcomes of Retinal Detachment Associated With Acute Retinal Necrosis. Ophthalmic Surg Lasers Imaging Retina 2016;47:660-4. [Crossref] [PubMed]
- Dave VP, Pappuru RR, Pathengay A, et al. Vitrectomy with Silicone Oil Tamponade in Rhegmatogenous Retinal Detachment following Acute Retinal Necrosis: Clinical Outcomes and Prognostic Factors. Semin Ophthalmol 2019;34:47-51. [Crossref] [PubMed]
- Blumenkranz MS, Culbertson WW, Clarkson JG, et al. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology 1986;93:296-300. [Crossref] [PubMed]
- Baltinas J, Lightman S, Tomkins-Netzer O. Comparing Treatment of Acute Retinal Necrosis With Either Oral Valacyclovir or Intravenous Acyclovir. Am J Ophthalmol 2018;188:173-80. [Crossref] [PubMed]
- Huynh TH, Johnson MW, Comer GM, et al. Vitreous penetration of orally administered valacyclovir. Am J Ophthalmol 2008;145:682-6. [Crossref] [PubMed]
- Flaxel CJ, Yeh S, Lauer AK. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2013;111:133-44.
- Wong R, Pavesio CE, Laidlaw DA, et al. Acute retinal necrosis: the effects of intravitreal foscarnet and virus type on outcome. Ophthalmology 2010;117:556-60. [Crossref] [PubMed]
- Dokey AT, Haug SJ, McDonald HR, et al. Acute retinal necrosis secondary to multidrug-resistant herpes simplex virus 2 in an immunocompetent adolescent. Retin Cases Brief Rep 2014;8:260-4. [Crossref] [PubMed]
- Stryjewski TP, Scott NL, Barshak MB, et al. Treatment of Refractory Acute Retinal Necrosis with Intravenous Foscarnet or Cidofovir. Ocul Immunol Inflamm 2018;26:199-203. [Crossref] [PubMed]
- Khurana RN, Charonis A, Samuel MA, et al. Intravenous foscarnet in the management of acyclovir-resistant herpes simplex virus type 2 in acute retinal necrosis in children. Med Sci Monit 2005;11:CS75-8. [PubMed]
- Bischoff-Jung M, Viestenz A, Fiorentzis M, et al. Intravitreales Ganciclovir als additive Therapieoption bei akuter retinaler Nekrose. Der Ophthalmologe 2017;114:838-42. [Crossref] [PubMed]
- Patel CV, Kishore K. Concomitant Intravitreal Ganciclovir and Dexamethasone Therapy in the Management of Acute Retinal Necrosis in a Patient Previously Treated with Oral Famciclovir. Case Rep Ophthalmol Med 2017;2017:4613624 [Crossref] [PubMed]
- Bauer D, Alt M, Dirks M, et al. A Therapeutic Antiviral Antibody Inhibits the Anterograde Directed Neuron-to-Cell Spread of Herpes Simplex Virus and Protects against Ocular Disease. Front Microbiol 2017;8:2115. [Crossref] [PubMed]
- Bauer D, Keller J, Alt M, et al. Corrigendum to "Antibody-based immunotherapy of acyclovir resistant ocular herpes simplex virus infection" Virology 2019;526:231-2. [Virology 512 (2017) 194-200]. [Crossref] [PubMed]
- Weissman HM, Biousse V, Schechter MC, et al. Bilateral central retinal artery occlusion associated with herpes simplex virus-associated acute retinal necrosis and meningitis: case report and literature review. Ophthalmic Surg Lasers Imaging Retina 2015;46:279-83. [Crossref] [PubMed]
- Yeh S, Fahle G, Flaxel CJ, et al. Central retinal vascular occlusion associated with acute retinal necrosis. Arch Ophthalmol 2012;130:514-7. [Crossref] [PubMed]
- Shantha JG, Weissman HM, Debiec MR, et al. Advances in the management of acute retinal necrosis. Int Ophthalmol Clin 2015;55:1-13. [Crossref] [PubMed]
- Majumder PD, Biswas J, Ambreen A, et al. Intravitreal dexamethasone implant for the treatment of cystoid macular oedema associated with acute retinal necrosis. J Ophthalmic Inflamm Infect 2016;6:49. [Crossref] [PubMed]
- Thrane AS, Hove M, Kjersem B, et al. Acute retinal necrosis and ocular neovascularization caused by cytomegalovirus following intravitreal dexamethasone implant (Ozurdex®) in an immunocompetent patient. Acta Ophthalmologica 2016;94:e813-4. [Crossref] [PubMed]
- Ando F, Kato M, Goto S, et al. Platelet function in bilateral acute retinal necrosis. Am J Ophthalmol 1983;96:27-32. [Crossref] [PubMed]
- Lau CH, Missotten T, Salzmann J, et al. Acute retinal necrosis features, management, and outcomes. Ophthalmology 2007;114:756-62. [Crossref] [PubMed]
- Sternberg P Jr, Han DP, Yeo JH, et al. Photocoagulation to prevent retinal detachment in acute retinal necrosis. Ophthalmology 1988;95:1389-93. [Crossref] [PubMed]
- Sims JL, Yeoh J, Stawell RJ. Acute retinal necrosis: a case series with clinical features and treatment outcomes. Clin Exp Ophthalmol 2009;37:473-7. [Crossref] [PubMed]
- Risseeuw S, de Boer JH, Ten Dam-van Loon NH, et al. Risk of Rhegmatogenous Retinal Detachment in Acute Retinal Necrosis With and Without Prophylactic Intervention. Am J Ophthalmol 2019;206:140-8. [Crossref] [PubMed]
- Hillenkamp J, Nölle B, Bruns C, et al. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology 2009;116:1971-5.e2. [Crossref] [PubMed]
- Luo YH, Duan XC, Chen BH, et al. Efficacy and necessity of prophylactic vitrectomy for acute retinal necrosis syndrome. Int J Ophthalmol 2012;5:482-7. [PubMed]
- Huang JM, Callanan P, Callanan D, et al. Rate of Retinal Detachment after Early Prophylactic Vitrectomy for Acute Retinal Necrosis. Ocul Immunol Inflamm 2018;26:204-7. [Crossref] [PubMed]
- Liu S, Wang D, Zhang X. The necessity and optimal time for performing pars plana vitrectomy in acute retinal necrosis patients. BMC Ophthalmol 2018;18:15. [Crossref] [PubMed]
- Ishida T, Sugamoto Y, Sugita S, et al. Prophylactic vitrectomy for acute retinal necrosis. Jpn J Ophthalmol 2009;53:486-9. [Crossref] [PubMed]
- . Oral acyclovir for herpes simplex virus eye disease: effect on prevention of epithelial keratitis and stromal keratitis. Herpetic Eye Disease Study Group. Arch Ophthalmol 2000;118:1030-6. [Crossref] [PubMed]
Cite this article as: Anthony CL, Bavinger JC, Yeh S. Advances in the diagnosis and management of acute retinal necrosis. Ann Eye Sci 2020;5:28.


