
Cytoprotective effect of amniotic membrane extracts on human corneal epithelial cells exposed to benzalkonium chloride in vitro
Introduction
Dry eye disease (DED) is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles (1). Ocular irritation, stinging and foreign body sensation are symptoms frequently reported by patients which are exacerbated by substance such as benzalkonium chloride (BAC) (2).
Most of eye drops contain preservatives to prevent potential contamination, and the most common preservative used is BAC, which is present in eye drops generally ranges from 0.004% to 0.02% (3). BAC is cytotoxic to bacteria and to ocular surface epithelial cells. Therefore, there are a number of reports on the development of ocular surface disorders (OSD) in patients treated with BAC containing eye drops. BAC may induce ocular discomfort, tear film instability, superficial structure and integrity impairment, conjunctival inflammation, subconjunctival fibrosis, and epithelial apoptosis (2,4-8). The toxicity of BAC on cultivated corneal and conjunctival cells have been evaluated in many different species. It induces cytoplasmic damage and apoptosis, and induce DNA strand-breakage and mitochondrial dysfunction (4). BAC has been employed to investigate the pathogenesis of DED in animal models (9). In rabbits, topically applied 0.1% BAC twice daily over 4–14 days results in the development of DED (10,11). In mice, 0.2% BAC applied twice daily triggers clinical signs of DED including loss of conjunctival goblet cells (GCs), increased fluorescein staining, and corneal irregularity (5,12-14).
Amniotic membrane (AM) is avascular and a rich source of biologically active factors with low immunogenicity and as such, promotes healing and acts as an effective material for wound dressing. AME supports epithelialization and exhibits anti-fibrotic, anti-inflammatory, anti-angiogenic and anti-microbial features (5). In ophthalmology, AM and amniotic membrane extract (AME) are widely used for ocular surface reconstruction, including the treatment of persistent epithelial defects and non-healing corneal ulcers, corneal perforations and descemetoceles, bullous keratopathy, as well as corneal disorders with associated limbal stem cell deficiency, pterygium, conjunctival reconstruction, corneoscleral melts and perforations, and glaucoma surgeries (15). Compared to AM, AME can be conveniently applied to the ocular surface without the risk of suture-related complications of amniotic membrane transplantation (AMT) (5). Boiled AME has also been successfully used to ameliorate dry eye induced by BAC in a murine model (5). However, the boiled avenue may destroy some bioactive factors, such as proteins may be destroyed and modified. Although there are numbers of study of BAC toxicity on human corneal epithelial cells (HCECs), there is minimal information regarding the preclinically protective effect of AME on HCECs exposed to BAC.
Based on these beneficial effects of AM on the ocular surface, we hypothesized that AME might protect the corneal epithelium from BAC-induced toxicity. To test the hypothesis, we investigated the effects of 10% AME in cultures of BAC-damaged HCECs and its possible mechanisms.
Methods
Preparation of AME
The human tissues were collected and used after written consent and approval from the Second Affiliated Hospital of Guangzhou Medical University were obtained. The placent AME were obtained from healthy women with no infectious diseases including human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and with an uncomplicated pregnancy undergoing an elective cesarean to term (38–40 weeks’ gestation). The placent AME were washed with sterile normal saline and the AM was manually stripped from the chorion. After the AM was separated, it was washed 3 times in phosphate-buffered saline (PBS) with antibiotics (penicillin 50 mg/L, streptomycin 50 mg/L, neomycin 100 mg/L, amphotericin B) under aseptic conditions for approximately 15 minutes each time. After soaking in sterile saline at 2.5 mg/L for 10–20 minutes, the AM was added to an equal volume of PBS solution in the homogenizer, ground, and centrifuged at 1,000 rpm for 5 min to obtain AME. AME was acquired with a 0.22-µm filter and then stored in a −20 °C refrigerator. The protein content of the AME was measured by the Folin-phenol method.
Cell culture
HCEC lines were obtained from a corneal epithelial cell line spontaneously derived from human limbal tissue through serial culture (SD-HCEC1s) from the research group of Professor Wang Zhichong of Zhongshan Ophthalmic Center, Sun Yat-sen University (16). Thirty cell passages were utilized in this study. The cells were seeded at a density of 500/cm2 in a 6-well plate (approximately 5,000 cells/well), and cultured in a 37 °C, 5% CO2 incubator.
When the cultured cells reached 70% confluence, the culture media were removed. Cells were incubated with BAC for 1 h at concentrations of 0.02% (the concentration was determined by the inhibition concentration 50 test based on the pre-experiment). To determine whether AME has the protective effects on BAC-damaged HCECs, AME at different concentrations of 50%, 20%, 10%, 2%, 1% were added to the cultures.
The experiment was divided into the following five groups: normal control (NC), NC + AME, BAC treatment untreated group (BAC), BAC treatment withdrawal (BAC + NC), BAC treatment withdrawal + AME group (BAC + AME), and NC + AME group.
Apoptosis analysis
Apoptosis and cell death were detected by flow cytometry on floating and adherent cells using an apoptosis detection kit (V13241, Invitrogen, Carlsbad, CA, USA). The cells were collected, washed once with cold PBS, centrifuged at 1,000 rpm for 5 min, resuspended in 1× annexin binding buffer, and then incubated with Annexin V-antibody and propidium iodide (PI). After dark incubation for approximately 1 h, the final suspension was analyzed using a FACS Calibur flow cytometer (BD Biosciences, Shanghai, China) and the results were analyzed using CellQuest Pro software (BD Biosciences, Shanghai, China).
Gene expression analysis
Total RNA extraction (n=3) was performed using TRIzol Reagent (Invitrogen). The number of cells used in each group was approximately 106.The extraction method was carried out according to the instructions and quantified according to the method described previously (17). The primers were shown in Table 1. First, single-stranded cDNA was synthesized from 1 µg of total RNA using the SYBR Prime ScriptTM RT-PCR kit (DRR063S. Takara, Dalian, China), following the instructions. The mRNA expressions of caspase-8, Muc1, Muc4, Muc16, Matrix-metalloproteinases (MMPs)-1, -2, -3, -8, -9, -13 CXCL1, IL-1, and IL-6 were detected by the SYBR green system (DRR063S.Takara, Dalian, China) with real-time PCR. The cycling conditions were the following: an initial denaturation temperature of 95 °C for 2 min, then 95 °C for 10 s, then 60 °C for 34 s (fluorescence signal acquisition), then 72 °C for 30 s for a total of 45 cycles, and then lastly, a temperature increase from 60 °C to 98 °C to obtain a melting curve. The results were analyzed by the cycle threshold (CT) method and the housekeeping gene GAPDH was used as a reference.
Table 1
| Gene | Sense primer | Antisense primer | Tm ( |
Product (bp) |
|---|---|---|---|---|
|
|
AGAGTCTGTGCCCAAATCAAC | GCTGCTTCTCTCTTTGCTGAA | 58 | 78 |
|
|
TGCCGCCGAAAGAACTACG | TGGGGTACTCGCTCATAGGAT | 60 | 76 |
|
|
GGAGAGGTATCGCCCTGATAG | CCGGTGTAGCCTGTAGAACTG | 59 | 76 |
|
|
CCAGTCCTACATCTTCGGTTGT | AGGGTAGTTCCTAGAGGGAGTT | 60 | 161 |
|
|
AAAATTACACGCCAGATTTGCC | GGTGTGACATTACTCCAGAGTTG | 59 | 82 |
|
|
CTGGACTCCGACACTCTGGA | CAGGAAAGGTTCTGAAGTGACC | 60 | 79 |
|
|
TGCTCTTACTCCATGTGCAGA | TCCAGGTAGTCCTGAACAGTTT | 59 | 85 |
|
|
ACTGAGAGGCTCCGAGAAATG | GAACCCCGCATCTTGGCTT | 60 | 103 |
|
|
ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA | 59 | 132 |
|
|
CCTGAACCTTCCAAAGATGGC | TTCACCAGGCAAGTCTCCTCA | 61 | 75 |
|
|
TGGCTTAGAACAAAGGGGCTTA | AAAGGTAGCCCTTGTTTCCC | 59 | 110 |
|
|
CAGCCTCTTCTCCTTCCTGAT | ggcccaggcagtcagat ca | 59 | 139 |
RT-PCR, real-time polymerase chain reaction.
Western blot analysis
According to previous studies (16,17), different groups of cells (approximately 107 cells in each group) were collected for western blot analysis. Cells at passage 30 were used to detect the expressions of the related proteins. The primary antibodies used were mouse anti-human caspase-8 (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-human Muc1 (1:100, Abcam, Cambridge, UK), mouse anti-human Muc4 (1:100, Abcam, Cambridge, UK), and mouse anti-human Muc16 (1:100, Abcam, Cambridge, UK). β-actin (1:3,000, Cell signaling, USA) was used as positive control. The secondary antibodies used were horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) and goat anti-rabbit IgG (Sigma-Aldrich, St. Louis, MO). Localization of antibodies was detected by a chemical luminescence using a ECL kit (Amersham, USA) following the manufacturer’s instructions. The images were analyzed using the Gel-Pro analyzer (Media Cybernetic, Inc.) for statistical analysis.
The expression of inflammatory cytokines by an enzyme-linked immunosorbent assay (ELISA)
ELISA (Biosource, San Francisco, CA, USA) were employed to detect the expression of MMP-1, -3, -8, -13, CXCL1, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α. ELISA procedures were performed following the manufacturer’s protocol.
The NC + 10% AME group was treated with IL-1β (0.1, 1.0, or 10.0 ng/mL) or TNF-α (0.1, 1.0, or 10.0 ng/mL) for 24 h to analyze the activity levels of MMP-8 protein in the supernatants of SD-HCEC1s cells. After that, the supernatants were collected and centrifuged before assays. The supernatants, pro-MMP-8 standard, and assay buffer were incubated in the 96-well plates which were pre-coated with anti-MMP-8 antibody at 4 °C for 12 hours. Then, the activity levels of MMP-8 protein were assayed using the commercial kits (Biotrak, Amersham).
Statistical analysis
All values are presented as mean ± standard deviation (SD). All analyses were evaluated with the analysis of variance followed by Boferroni’s post hoc test using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA). In cases of nonparametric data distribution, the Kruskal-Wallis test was performed, followed by the Mann-Whitney U test. A probability value of 0.05 (P<0.05) was considered to be statistically significant.
Results
AME enhances cell viability and inhibits apoptosis of SD-HCEC1s treated with BAC
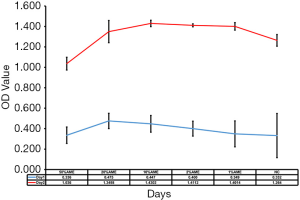
As shown in Figure 1, cell proliferation assays showed that SD-HCEC1s in 10% AME possessed relatively strong growth capacities as compared to those in the 50% AME, 20% AME, 2% AME, 1% AME, and NC groups. These results showed that the SD-HCEC1s possessed a higher proliferative capacity in 10% AME after withdrawal of the 0.02% BAC.
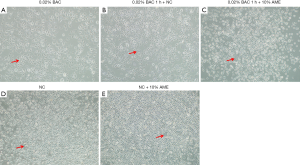
Morphologically, the SD-HCEC1s treated with 0.02% BAC exhibited relatively more vacuolated cells (Figure 2). The SD-HCEC1s in the 0.02% BAC 1 h + 10% AME group exhibited relatively lower vacuolated cells than the cells in the 0.02% BAC group.
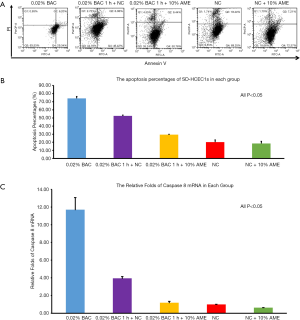
Flow cytometry revealed that the percentage of apoptotic cells in the 0.02% BAC, 0.02% BAC 1 h + NC, 0.02% BAC 1 h + 10% AME, NC, NC + 10% AME groups were 74.13%±4.00%, 52.60%±1.68%, 29.52%±0.72%, 20.13%±5.04%, 18.46%±4.73%, respectively. Compared to the continuously cultured 0.02% BAC group, the 0.02% BAC 1 h + 10% AME possessed fewer apoptotic cells (P<0.001, n=3) (Figure 3A,B). Compared to the NC groups, SD-HCEC1s cultured in the NC + 10% AME groups exhibited fewer apoptotic cells (P<0.001, n=3).
AME downregulates the expression of caspase-8 and enhances the maintenance of the functional properties of mucins in SD-HCEC1s
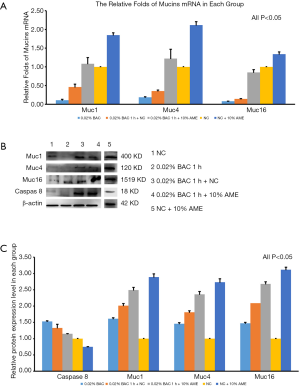
The mRNA expression of caspase-8 was significantly increased after adding 0.02% BAC to SD-HCEC1s for 1 h. However, the level of mRNA transcripts for caspase-8 was detected at lower levels in the 0.02% BAC 1 h + 10% AME group (P<0.001, n=3) (Figure 3C). The mRNA expression levels of Muc1, Muc4, and Muc16 were significantly decreased after adding 0.02% BAC for 1 h (P<0.05, n=3), while the mRNA levels of Muc1, Muc4, and Muc16 were dramatically increased (P<0.01, n=3) in 0.02% BAC 1 h + 10% AME group (Figure 4A). Western blot showed that the protein expression of caspase-8 was significantly increased in 0.02% BAC group (P<0.05, n=3). The protein expression levels of Muc1, Muc4, and Muc16 were significantly decreased after SD-HCEC1s were exposed to BAC, while the protein expression of Muc1, Muc4 and Muc16 were dramatically increased in the 0.02% BAC 1 h + 10% AME group (P<0.01, n=3) (Figure 4B,C).
To evaluate the effect of AME on the regulation of the inflammatory cytokines in SD-HCEC1s exposed to BAC, cytokines MMP-1, MMP-3, MMP-8, MMP-13, CXCL1, IL-1β, IL-6, and TNF-α were examined. Compared to the cells exposed to 0.02% BAC, the mRNA expression levels of MMP-1, -3, -13, IL-1, IL-6, TNF-α were significantly reduced in 0.02% BAC 1 h + 10% AME group (P<0.01, n=3). Interestingly, the expression levels of MMP-8 were increased in 0.02% BAC 1 h + 10% AME group (P<0.01, n=3) (Table 2). The concentrations of MMP-1, MMP-3, MMP-13, CXCL1, IL-1β, IL-6, and TNF-α proteins were significantly higher in the 0.02% BAC group than the cells cultured in both the NC group (P<0.001, n=3) and 0.02% BAC 1 h + 10% AME group (P<0.05, n=3). MMP-8 levels in NC + 10% AME were higher than the cells in NC group (P<0.001, n=3). Compared to NC group, the concentrations of MMP-1, MMP-3, MMP-13, CXCL1, IL-1β, IL-6, and TNF-α proteins in NC + 10% AME were significantly lower (P<0.001, n=3) (Table 3).
Table 2
| Variables | 0.02% BAC | 0.02% BAC 1 h + NC | 0.02% BAC 1 h + 10% AME | NC | NC + 10% AME |
|---|---|---|---|---|---|
| MMP-1 | 1.28±0.10a | 1.12±0.06d | 0.85±0.03b | 1.00±0.01c | 0.73±0.03e |
| MMP-3 | 1.37±0.11a | 1.13±0.09d | 0.86±0.04b | 1.00±0.01c | 0.85±0.03e |
| MMP-8 | 0.84±0.02 | 0.90±0.04d | 1.07±0.02b | 1.00±0.01c | 1.34±0.04e |
| MMP-13 | 1.32±0.07a | 1.06±0.09 | 0.91±0.07b | 1.00±0.01c | 0.68±0.03e |
| CXCL1 | 1.06±0.08a | 0.85±0.07 | 0.76±0.04b | 1.00±0.01 | 0.53±0.03e |
| IL-1β | 1.29±0.06a | 0.94±0.08 | 0.88±0.09b | 1.00±0.01c | 0.62±0.07e |
| IL-6 | 1.26±0.07 | 1.21±0.08d | 1.01±0.05b | 1.00±0.01c | 0.71±0.04e |
| TNF-α | 1.19±0.06a | 0.84±0.08 | 0.78±0.09b | 1.00±0.01c | 0.52±0.07e |
a, P<0.05 0.02% BAC
Table 3
| Variables | 0.02% BAC | 0.02% BAC 1 h + NC | 0.02%BAC 1 h + 10% AME | NC | NC + 10% AME |
|---|---|---|---|---|---|
| MMP-1 (ng/mL) | 77.01±6.00a | 40.32±2.16d | 20.40±0.72b | 19.00±0.19c | 17.21±0.17e |
| MMP-3 (ng/mL) | 73.77±5.94a | 36.61±2.92d | 18.58±0.86b | 17.10±0.17c | 15.52±0.15e |
| MMP-8 (ng/mL) | 28.50±0.29a | 33.48±1.80d | 58.32±2.59b | 53.81±1.28c | 60.13±1.44e |
| MMP-13 (pg/mL) | 75.42±7.80a | 38.16±3.24d | 21.84±1.68b | 19.00±0.19c | 17.21±0.17e |
| CXCL1 (ng/mL) | 47.57±3.60a | 23.02±1.89d | 13.74±0.72b | 14.25±0.14c | 12.98±0.13e |
| IL-1β (ng/mL) | 77.54±3.60a | 33.84±2.88d | 21.12±2.16b | 19.00±0.19c | 17.21±0.17e |
| IL-6 (ng/mL) | 94.50±5.25a | 54.26±3.60d | 30.30±1.50b | 23.75±0.24c | 21.44±0.21e |
| TNF-α (ng/mL) | 53.66±2.70a | 22.68±2.16d | 14.04±1.62b | 12.83±0.14c | 11.71±0.13e |
a, P<0.05 0.02% BAC
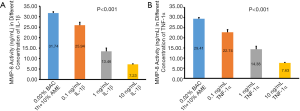
MMP-8 activity in the conditioned media was significantly reduced after treatment with 0.1, 1, and 10 ng/mL of IL-1β, respectively. Similarly, MMP-8 activity was inhibited by TNF-α. The concentrations of MMP-8 in the control group, 0.1, 1, and 10 ng/mL of TNF-α were 29.49±0.59, 22.74±1.31, 14.38±1.46, and 7.63±0.23 ng/mL, respectively (Figure 5).
Discussion
The current study demonstrated that BAC decreased HCECs viability and proliferation, and increased cellular apoptosis. Addition of 10% AME remarkably protected HCECs against BAC-induced toxicity. The key finding is that the protective effect of AME on SD-HCEC1s exposed to BAC was mediated partially by upregulation of MMP-8 and downregulation of IL-1β and TNF-α.
DED is well documented that typical pathologic alterations of dry eye involve ocular surface inflammation, conjunctival GC loss, corneal epithelium squamous metaplasia, and epithelial disruption. In DED, the inflammations may play vital roles (18,19). IL-1β and TNF-α both play important roles in the mechanisms of DED (20,21). IL-6 plays a vital role in the induction of acute inflammation which is generated by macrophages and T cells. Bian et al. found that an increase in the expressions and activities of MMPs-1, -2, -3, -8, -9, and -13 were positively associated with the severity of corneal diseases. Additionally, in corneal wound healing procedures, the generations and activities of MMPs have elevated (22-24).
BAC has been the most common preservative used in eye drops, especially in anti-glaucoma medications (6,7). BAC acts as a surfactant to solubilize ionic components into immiscible solvents, which facilitates effective emulsification and stabilization of medications, and prolongs shelf-life. Futher, BAC is superior to other preservatives in inhibiting microbial activity (3,25,26). The half-life of BAC is approximately 20 hours in the corneal epithelium and conjunctiva, which may lead to chronic ocular surface damage in patients using BAC-containing eye drops (3,5,27). Prolonged exposure to BAC caused toxicity to the ocular surface. Preservative-free eye drops are the ultimate choice for ocular surface disease. However, preservative eye drops had a risk of microbial contamination when used more than once over a period of 10 hours. It is critical to manage BAC-induced ocular surface toxicity. Our study showed that AME had beneficial effects in preventing and treating BAC-induced toxicity in HCECs (27).
AM can inhibit the release of inflammatory mediators and their matrix surface can also capture inflammatory cells, leading to anti-inflammatory effects when covering the ocular surface (15,28-30). AME, the extracted purified component of AM, has unique biological characteristics. Advantage of the application of AME is that it not only possesses the effective components of AM but also avoids the suture-related risk. It is generally believed that AME has effective components, including HA-HC complex (hyaluronic and the heavy chains of inter-α-inhibitor, which are responsible for anti-inflammatory, and anti-scarring), fibronectins, peptides, small proteins, and growth factors (15,31-33). Unlike boiled method, we employed homogenized and filtered method to acquire AME, which can possess almost all the effective components of AM maximally. This work demonstrated that AME protected SD-HCEC1s when stressed in BAC via upregulation of MMP-8 and downregulation of IL-1β and TNF-α.
In summary, this study suggested that AME provided protection for SD-HCEC1s exposed to BAC. AME maintained the normal function of SD-HCEC1s by increasing cellular activity, inhibiting apoptosis, and maintaining mucin secretion via upregulation of MMP-8 and downregulation of IL-1β and TNF-α. AME may have the potential to be employed as a topical adjunctive therapy in eyes chronically exposed to BAC.
Acknowledgments
Funding: This study was supported by the “Yangcheng Scholar” Youth Research Backbone Training Project of Guangzhou Municipal College (No. 1201581612), Guangzhou Science and Technology Project (No. 201804010038), and Guangdong Natural Science Foundation of China (2020A1515010276; No. 20 15A030313479).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2020.02.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The human tissues were collected and used after written consent and approval from the Second Affiliated Hospital of Guangzhou Medical University were obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf 2017;15:276-83. [Crossref] [PubMed]
- Baudouin C, Labbe A, Liang H, et al. Preservatives in eye drops: the good, the bad and the ugly. Prog Retin Eye Res 2010;29:312-34. [Crossref] [PubMed]
- Pisella PJ, Fillacier K, Elena PP, et al. Comparison of the effects of preserved and unpreserved formulations of timolol on the ocular surface of albino rabbits. Ophthalmic Res 2000;32:3-8. [Crossref] [PubMed]
- Ye J, Wu H, Zhang H, et al. Role of benzalkonium chloride in DNA strand breaks in human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol 2011;249:1681-7. [Crossref] [PubMed]
- Xiao X, Luo P, Zhao H, et al. Amniotic membrane extract ameliorates benzalkonium chloride-induced dry eye in a murine model. Exp Eye Res 2013;115:31-40. [Crossref] [PubMed]
- Rasmussen CA, Kaufman PL, Kiland JA. Benzalkonium chloride and glaucoma. J Ocul Pharmacol Ther 2014;30:163-9. [Crossref] [PubMed]
- Rahmatnejad K, Rapuano CJ, Ichhpujani P, et al. The Effects of Latanoprost With Benzalkonium Chloride Versus Travoprost With SofZia on the Ocular Surface. Eye Contact Lens 2018;44:S93-S98. [Crossref] [PubMed]
- Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride- and polyquad-preserved combination glaucoma medications on cultured human ocular surface cells. Adv Ther 2011;28:501-10. [Crossref] [PubMed]
- Zhang R, Park M, Richardson A, et al. Dose-dependent benzalkonium chloride toxicity imparts ocular surface epithelial changes with features of dry eye disease. Ocul Surf 2020;18:158-69. [Crossref] [PubMed]
- Xiong C, Chen D, Liu J, et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Invest Ophthalmol Vis Sci 2008;49:1850-6. [Crossref] [PubMed]
- Chen W, Li Z, Hu J, et al. Corneal alternations induced by topical application of benzalkonium chloride in rabbit. PLoS One 2011;6:e26103 [Crossref] [PubMed]
- Lin Z, Liu X, Zhou T, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis 2011;17:257-64. [PubMed]
- Zhang Z, Yang WZ, Zhu ZZ, et al. Therapeutic effects of topical doxycycline in a benzalkonium chloride-induced mouse dry eye model. Invest Ophthalmol Vis Sci 2014;55:2963-74. [Crossref] [PubMed]
- Xiao X, He H, Lin Z, et al. Therapeutic effects of epidermal growth factor on benzalkonium chloride-induced dry eye in a mouse model. Invest Ophthalmol Vis Sci 2012;53:191-7. [Crossref] [PubMed]
- Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank 2017;18:193-204. [Crossref] [PubMed]
- Liu J, Song G, Wang Z, et al. Establishment of a corneal epithelial cell line spontaneously derived from human limbal cells. Exp Eye Res 2007;84:599-609. [Crossref] [PubMed]
- Liu Z, Zhan W, Zeng M, et al. Enhanced functional properties of human limbal stem cells by inhibition of the miR-31/FIH-1/P21 axis. Acta Ophthalmol 2017;95:e495-e502. [Crossref] [PubMed]
- Huber S, Gagliani N, Esplugues E, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011;34:554-65. [Crossref] [PubMed]
- Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol 2009;2:375-6. [Crossref] [PubMed]
- Liu R, Ma B, Gao Y, et al. Tear Inflammatory Cytokines Analysis and Clinical Correlations in Diabetes and Nondiabetes With Dry Eye. Am J Ophthalmol 2019;200:10-5. [Crossref] [PubMed]
- Shivakumar S, Panigrahi T, Shetty R, et al. Chloroquine Protects Human Corneal Epithelial Cells from Desiccation Stress Induced Inflammation without Altering the Autophagy Flux. Biomed Res Int 2018;2018:7627329 [Crossref] [PubMed]
- Bian F, Wang C, Tukler-Henriksson J, et al. MMP-8 Is Critical for Dexamethasone Therapy in Alkali-Burned Corneas Under Dry Eye Conditions. J Cell Physiol 2016;231:2506-16. [Crossref] [PubMed]
- Lyu J, Joo CK. Expression of Wnt and MMP in epithelial cells during corneal wound healing. Cornea 2006;25:S24-8. [Crossref] [PubMed]
- Sakimoto T, Sawa M. Metalloproteinases in corneal diseases: degradation and processing. Cornea 2012;31:S50-6. [Crossref] [PubMed]
- Kim MS, Choi CY, Kim JM, et al. Microbial contamination of multiply used preservative-free artificial tears packed in reclosable containers. Br J Ophthalmol 2008;92:1518-21. [Crossref] [PubMed]
- Charnock C. Are multidose over-the-counter artificial tears adequately preserved? Cornea 2006;25:432-7. [Crossref] [PubMed]
- Moon J, Ko JH, Yoon CH, et al. Effects of 20% Human Serum on Corneal Epithelial Toxicity Induced by Benzalkonium Chloride: In Vitro and Clinical Studies. Cornea 2018;37:617-23. [Crossref] [PubMed]
- Evron A, Goldman S, Shalev E. Human amniotic epithelial cells cultured in substitute serum medium maintain their stem cell characteristics for up to four passages. Int J Stem Cells 2011;4:123-32. [Crossref] [PubMed]
- Liang X, Liu Z, Lin Y, et al. A modified symblepharon ring for sutureless amniotic membrane patch to treat acute ocular surface burns. J Burn Care Res 2012;33:e32-e38. [Crossref] [PubMed]
- Sha X, Liu Z, Song L, et al. Human amniotic epithelial cell niche enhances the functional properties of human corneal endothelial cells via inhibiting P53-survivin-mitochondria axis. Exp Eye Res 2013;116:36-46. [Crossref] [PubMed]
- He H, Li W, Tseng DY, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem 2009;284:20136-46. [Crossref] [PubMed]
- Shay E, He H, Sakurai S, et al. Inhibition of angiogenesis by HC.HA, a complex of hyaluronan and the heavy chain of inter-alpha-inhibitor, purified from human amniotic membrane. Invest Ophthalmol Vis Sci 2011;52:2669-78. [Crossref] [PubMed]
- Liang L, Li W, Ling S, et al. Amniotic membrane extraction solution for ocular chemical burns. Clin Exp Ophthalmol 2009;37:855-63. [Crossref] [PubMed]
Cite this article as: Liu J, Zou H, Zeng M, Huang AM, Chen Y, Han E, Sha X, Liu Z. Cytoprotective effect of amniotic membrane extracts on human corneal epithelial cells exposed to benzalkonium chloride in vitro. Ann Eye Sci 2020;5:11.


