
Retinal damage after exposure to white light emitting diode lights at different intensities in Sprague-Dawley rats
Introduction
The retina plays a crucial role in the visual system by receiving light signals and converting them into electrical signals, but extensively high intensity and/or long duration of light exposure will lead to retinal photodamage (1-3). Human beings have lived on natural light since ancient times by following and adapting to the circadian rhythms and natural light-dark cycle. However, the occurrence of artificial light sources with the development of science and technology has dramatically changed the basic biological rhythm of day and night. A variety of light sources, when misused, may cause damage to the human retina. In 1966, Noell et al. (4) established the first rat model of retinal photodamage. Subsequently, many experiments have been performed on light damage in different animals under different injury conditions.
In recent years, light emitting diode (LED) light sources including mobile phones, desk lamps, home appliances, and lighting products for public lighting and industrial production have been made widely available due to LED’s many advantages like low DC voltage input, low energy consumption, broad applicability, high stability, lack of stroboscopic effect, short response time, and multi-color illumination. However, the impact of LED lights on the retina remains unclear. In our current study, rats were exposed to LED lamps with different illumination intensities, and the results were compared with normal conditions and ultraviolet B (UVB) exposure at 302 nm. We observed and analyzed their effects on the function and morphology of rat retinas, with an attempt to guide the reasonable use of LED light sources.
Methods
Experimental light sources
Four commercially available LED lamps with different illumination intensities were selected, and their illumination intensity at the experimental distance was 4,000, 6,000, 7,000, and 10,000 lux, respectively, as measured by an illuminometer (Lightmeter, TES-1334A). Also, a UVB lamp with an intensity of 1,000 µw/cm2, which was detected by UV lightmeter (TenMars TM213), was used, with a working wavelength of 302 nm.
Laboratory animals and grouping
Thirty-six Sprague-Dawley rats (18 males and 18 females) weighing 150–180 g were purchased from Guangdong Provincial Medical Laboratory Animal Center [SCXK (Guangdong) 2018-0002]. These animals were randomly divided into six groups (n=6 in each group) including a normal control (NC) group; four white LED groups at different light intensities (4,000, 6,000, 7,000, and 10,000 lux), and a UVB lighting group.
Animal experiments were performed in a manner consistent with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Animal Ethics Committee of the Zhongshan Ophthalmology Center. The animals were housed in the Experimental Animal Center of Zhongshan Ophthalmology Center of Sun Yat-sen University. They were maintained under controlled light schedule (12 h light, 12 h dark) at room temperature (20–24 °C) and with constant humidity (55%). All animals received food and water ad libitum.
Modeling of LED light-induced retinal damage
All animals were adaptively fed for three days under normal conditions before light exposure to ensure the consistencies among the rats. Animals in the four LED groups were placed in an illumination box with IED lamps at different light intensities (4,000, 6,000, 7,000, and 10,000 lux) and exposed to the light for 24 h. Rats in the UVB group were placed in an illumination box with UVB lamp with a wavelength of 302 nm, an intensity of 1,000 µw/cm2, and illuminated for 24 h consecutively. Normal feeding conditions were maintained in the NC group, without illumination from any additional light source. All animals received food and water ad libitum during the illumination.
Whole-field flash electroretinogram (FERG)
FERG is currently the only examination that can objectively reflect retinal function by measuring retinal function layer by layer at the cellular level (5). Rats were dark-adapted for 30 min before the test and then anesthetized by intraperitoneal administration of 10% chloral hydrate (4 mL/kg). The pupils were dilated with compound tropicamide eye drops (Santen Pharmaceuticals Co. Ltd., Osaka, Japan). Ophthalmic anesthesia was induced with 0.5% tetracaine hydrochloride eye drops (Zhongshan Ophthalmic Center, Sun Yat-sen University), and hypromellose eye drops (Zhongshan Ophthalmic Center, Sun Yat-sen University) were applied to increase the electrical conductivity of rat eyes. Roland Consult visual electric physiological system and Color Ganzfeld Q450C stimulator were used for FERG, which obtained parameters including scotopic 0.01 ERG, scotopic 3.0 ERG, scotopic 10.0 ERG, scotopic 3.0 oscillatory potential ERG, and photopic 3.0 ERG. The recording electrode, reference electrode, and ground electrode were placed at the corneal surface, subcutaneous tissue of the lower eyelid, and subcutaneous tissue of the tail, respectively. After the test was completed, the a- and b-wave amplitudes and the a- and b-wave peak time were recorded in each group. The amplitude and peak time of the OP2 wave of the oscillating potential were also analyzed.
Pathology and light microscopy
Rats were sacrificed by cervical dislocation after ERG. Both eyeballs were surgically removed and fixed in 10% formalin for 48 h, dehydrated through an ethanol gradient, embedded in paraffin, and cut into serial sections. Light microscopy (Axioplan 2, Zeiss) was performed after hematoxylin and eosin (H&E) staining to observe the retinal damage.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software package. Measurement data are expressed as mean ± standard deviation (mean ± SD), and one-way ANOVA analyzed inter-group differences. Bonferroni correction or Dunnett T3 test was applied for multiple comparisons after the test for homogeneity of variances. A two-sided P value of <0.05 was considered statistically significant.
Results
FERG tests
Compared with the NC group, the b-wave peak time of scotopic 0.01 ERG showed no statistically significant change in four LED groups after 24 h illumination (Table 1) but significantly decreased in the UVB group (P<0.01). However, the b-wave amplitude of scotopic 0.01 ERG was significantly lower in all illumination groups when compared to the NC group (all P<0.01), and the decrease of the b-wave amplitude of scotopic 0.01 ERG was notably higher in the UVB group.
Table 1
| Group | Flash intensity | ||||||
|---|---|---|---|---|---|---|---|
| 0.01 | 3.0 | 10.0 | |||||
| b-wave time | a-wave time | b-wave time | a-wave time | b-wave time | |||
| NC | 90.88±10.77 | 17.22±3.35 | 69.67±9.11 | 15.47±2.46 | 77.14±15.62 | ||
| 4,000 | 96.30±5.04 | 19.40±1.44 | 65.29±6.05 | 18.03±1.68 | 69.53±5.16 | ||
| 6,000 | 99.26±24.74 | 22.87±2.43** | 70.33±12.23 | 21.03±2.15** | 65.88±12.60 | ||
| 7,000 | 97.59±8.96 | 20.48±1.89 | 59.69±8.85 | 19.47±2.37** | 58.78±8.16** | ||
| 10,000 | 89.48±8.17 | 21.90±3.67* | 58.44±15.21 | 19.83±3.81** | 45.87±6.63** | ||
| UVB | 55.85±18.69** | 29.62±1.95** | 80.68±7.56 | 29.41±2.41** | 75.04±6.60 | ||
*, P<0.05
Compared with the NV group, the a-wave peak time of scotopic 3.0 ERG was increased in all illumination groups (Table 1), and, in the UVB group particularly, there was significant difference between the 6,000 lux group (P<0.01) and the UVB group, and between the 10,000 lux group and the UVB group (P<0.05). Also, the b-wave peak time of scotopic 3.0 ERG showed no significant difference between the illumination groups and NC group (Table 1). Compared with the NC group, the a-wave amplitude of scotopic 3.0 ERG significantly decreased in all illumination groups (P<0.05 or P<0.01) (Table 2), and the b-wave amplitude of scotopic 3.0 ERG significantly decreased in all illumination groups except the 4,000 lux group (all P<0.05).
Table 2
| Group | Flash intensity | ||||||
|---|---|---|---|---|---|---|---|
| 0.01 | 3.0 | 10.0 | |||||
| b-wave amplitude | a-wave amplitude | b-wave amplitude | a-wave amplitude | b-wave amplitude | |||
| NC | 60.10 ±22.79 | 51.14±22.20 | 123.24±42.04 | 58.49±14.02 | 130.77±37.67 | ||
| 4,000 | 33.71±20.98** | 25.13±7.02* | 92.68±27.77 | 30.05±7.94** | 100.80±28.42 | ||
| 6,000 | 27.37±15.98** | 17.54±5.51** | 74.78±16.70* | 24.51±5.55** | 85.00±16.37* | ||
| 7,000 | 28.35±18.39** | 23.42±8.84* | 78.45±23.21* | 28.43±9.95** | 89.36±29.48* | ||
| 10,000 | 27.06 ±15.42** | 19.79±12.54** | 71.68±21.24* | 23.14±11.26** | 83.09±44.44** | ||
| UVB | 9.74±7.79** | 14.56±5.37** | 79.12±19.15* | 18.05±8.35** | 91.44±25.05* | ||
*, P<0.05; **, P<0.01, compared with the NC group. UNC, normal control.
Compared with the NC group, the a-wave peak time of scotopic 10.0 ERG also increased in all illumination groups (Table 1), in particular the UVB group. The changes of the a-wave peak time showed significant differences in all illumination groups except the 4,000 lux group (all P<0.01). The decrease in b-wave peak time in the 7,000 lux group and the 10,000 lux group was significantly different from that in the NC group (both P<0.01). Compared with the NC group, the a-wave amplitude of scotopic 10.0 ERG significantly decreased in all illumination groups (all P<0.01), especially in the UVB group; the b-wave amplitude of scotopic 10.0 ERG also reduced considerably in all illumination groups except the 4,000 lux group (P<0.05 or P<0.01), with the 10,000 lux group having the most significant decline.
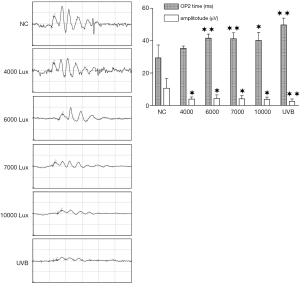
The results of the scotopic 3.0 oscillatory potential ERG are shown in Figure 1. The OP2-wave peak time in the NC group (29.35±7.79 ms) was not significantly different from that in the 4,000 lux group (35.34±1.38 ms) but was significantly different from those in the 6,000 lux group (41.54±2.54 ms), 7,000 lux group (41.16±3.73 ms), 10,000 lux group (40.18±4.93 ms), and UVB group (49.74±4.18 ms). Meanwhile, compared with the amplitude in the NC group (10.70±5.96 µV), the OP2-wave amplitude significantly decreased in the 4,000 lux group (3.90±1.33 µV), 6,000 lux group (4.28±2.24 µV), 7,000 lux group (4.07±1.94 µV), 10,000 lux group (3.70±1.47 µV), and UVB group (2.49±1.53 µV).
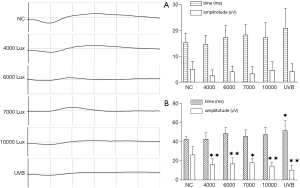
The amplitude and peak time of the a-wave of photopic 3.0 ERG showed no significant difference among all groups (Figure 2A). Compared with that in the NC group (42.51±3.32 ms), the b-wave peak time of photopic 3.0 ERG (Figure 2B) in the four LED groups including the 4,000 lux group (42.69±6.58 ms), 6,000 lux group (48.53±6.41 ms), 7,000 lux group (45.58±6.86 ms), and 10,000 lux group (47.35±7.77 ms) showed no significant difference but significantly increased in the UVB group (51.62±10.42 ms) (P<0.05); compared with that in the NC group (26.25±8.76 µV), the b-wave amplitude of photopic 3.0 ERG (Figure 2B) in the four LED groups including the 4,000 lux group (16.02±6.40 µV), 6,000 lux group (16.86±6.46 µV), 7,000 lux group (18.00±4.48µV), 10,000 lux group (14.50±4.25 µV) and in the UVB group, significantly declined (P<0.05 or P<0.01).
Pathological examination
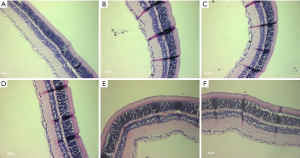
In the NC group (Figure 3A), the retina had typical morphologies featured by well-defined structures, clearly visible border between the inner and outer segments, and neatly arranged inner and outer nuclear layer cells. After 24 h of illumination, the inner and outer portions of the retina were still neatly arranged (Figure 3B), along with a clear border; however, the inner and outer nuclear layers were disordered, and some irregular nuclei and cells were lost. The inner and outer segments were randomly arranged in the 6,000 lux group (Figure 3C), 7,000 lux group (Figure 3D), and 10,000 lux group (Figure 3E); meanwhile, the arrangement of inner and outer nuclear layers became even more disordered, and the loss of cells was more noticeable. In the UVB group (Figure 3F), the disordered arrangement of inner and outer segments was obvious, along with the increased disorderedly arrangement of inner and outer nuclear layers and loss of cells.
Discussion
The retina is the most vulnerable part of the eye tissue to light damage, and Noell et al. (1,4) have found that light can cause damage to the retina. Jin et al. (5) found that the severity of retinal photodamage in rats exposed to a light cycle of low and moderate intensities was associated with light intensity and exposure duration. Lights of different wavelengths damage the retinal tissue via various mechanisms including thermal damage, mechanical damage, and photochemical damage, with the latter being the most common type. Photochemical damage is believed to be associated with peroxidation (6), rhodopsin-mediated reaction (7), calcium overload-mediated response (8), Mǜller cell-mediated response (9), and mechanisms that regulate cell apoptosis (10,11).
LED light sources are now widely used due to their excellent performance. White LED is usually formed in two ways. The first is the use of blue light technology to match the phosphor to create white light, and the second one is a variety of monochromatic light mixing methods (e.g., the combination of blue light, green light, and red light). Both ways require the use of blue light (12), which is quite harmful to the retina (11). In our current experiment, we observed the retinal damage after 24 h of exposure to LED lights at different illumination intensities (4,000, 6,000, 7,000, and 10,000 lux) or to UVB lamps at a wavelength of 302 nm and an intensity of 1,000 µw/cm2.
Retinal photodamage in our experiment was mainly assessed by FERG. Retinal waves are generated in different ways. The a-wave is believed to be caused by retinal cone and rod cells, representing the potential activity of photoreceptor cells in the outer nuclear layer of the retina; in contrast, the b-wave comes from the bipolar cells or the Mǜller cells in the retinal inner nuclear layer, representing the potential of the post-synaptic neurons in the inner nuclear layer. The oscillating potential is a series of low-amplitude potentials attached to the a- and b-waves. It is generated by the amacrine cells in the inner nuclear layer, reflecting the function of the inner layer of the retina and is related to the blood circulation of the retina. During FERG, scotopic 0.01 ERG is a rod-driven response that can reflect rod cell function, while scotopic 3.0 ERG is the combined response arising from photoreceptors and bipolar cells of both the rod and cone systems (rod-dominated). Scotopic 10.0 ERG is a combined response with enhanced a-waves reflecting photoreceptor function, scotopic oscillatory potentials are responses primarily from amacrine cells, and photopic 3.0 ERG is the response of the cone system. The a-waves arise from cone photoreceptors and off-cone bipolar cells and the b-wave comes from on- and off-cone bipolar cells. By analyzing the experimental results, we found that different light intensities and different types of light sources caused various degrees of damage to retinal cells. The degree of light damage to retinal function gradually increased with the rise of LED luminosity. In contrast, the UVB group had the most severe retinal function impairment. Notably, functional damage of rod cells and inner nuclear layer cells was the main FERG finding in each group.
Pathology after 24 h of illumination showed that the inner and outer segments of the retina were still neatly arranged, along with clear border; however, the inner and outer nuclear layers were disordered, and some irregular nuclei and cells were lost. The damage of the internal and external retinal segments and the internal and external nuclear layers was more evidentin the 6,000 lux group, 7,000 lux group, and 10,000 lux group. Compared with the LED groups, the UVB group had a more apparent disordered arrangement of inner and outer nuclear layers and loss of cells.
Penn et al. (7) also discovered a positive correlation between the retinal rhodopsin level and the susceptibility of the retina to photodamage. Jin et al. (5) found the cells on the retina decreased, and their arrangement became tight and irregular after extended exposure to low- and moderate-intensity white light (100–1,500 lux). Kremers et al. (13) summarized that there are two classes of retinal photodamage. Class I is found after low-intensity exposure, with retinal irradiance seldom exceeding 1 mW/cm2; after long-term white light irradiance, the damage is mainly restricted to the photoreceptor level. Class II is seen after short-term (<4 h), high-intensity (>10 mW/cm2) irradiance, with the first sign of damage appearing in the retinal pigment epithelium (RPE). In our current experiment, we mainly investigated the deterioration of the retina after medium-/high-intensity, medium-/long-duration exposure to white light, or ultraviolet light. In the research performed by Jaadane et al. (14), commercially available white LEDs and four different blue LEDs (507, 473, 467, and 449 nm) were used for exposure experiments on Wistar rats for 6 to 72 h. Immunohistochemical stain, transmission electron microscopy, and western blot were used to exam the retinas. It was found that the blue light caused the massive loss of retinal outer nuclear layer cells due to oxidative stress.
Therefore, the intensity and exposure duration of the LED light sources are closely related to the degree of damage to the retina (15). LED components are another concern. Full-spectrum light is considered an eye-protecting light source; however, it does not mean the availability of all lights but a high uniformity of light sources. A lower proportion of the integral value of the blue light over the total spectral integral value is more favorable for retina protection (12) although the exact value requires further investigation.
In summary, the continuous exposure to white LED light can cause structural and functional damage to rat retinas, and such damage is related to the intensity of illumination. Therefore, the risk of retinal damage should be considered during LED illumination, and proper LED illumination intensity may help to maintain eye health.
Acknowledgments
The authors thank all the participants in this study, without whom the research could not have been performed so smoothly. This work was conducted with support from the Guangzhou No. 16 Middle School and Department of Pharmacy of Zhongshan Ophthalmological Center.
Funding: Supported by the Guangdong Natural Science Foundation (2016A030313294).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2019.06.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Animal Ethics Committee of Zhongshan Ophthalmological Center of Sun Yat-sen University (ID: 2016-157). Animal experiments were performed in a manner consistent with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noell WK. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res 1980;20:1163-71. [Crossref] [PubMed]
- Zhao YP, Sun SY, Niu JJ. Progression of prevention and treatment of retinal light injury. Int J Ophthalmol 2009;9:2132-5.
- Chen P, Qian HW. Advances in the mechanisms of photochemical damage of the retina. Foreign Med Sci: Ophthalmol 2002;26:96-9.
- Noell WK, Walker VS, Kang KS, et al. Retinal damage by light in rats. Invest Ophthalmol 1966;5:450-73. [PubMed]
- Jin XM, Wu LZ, Zheng HL, et al. Retinal photodamage: I. The effects of light exposure time under medium- and low-intensity cyclic illumination. Eye Sciences 1998;14:215-9.
- Chen E. Inhibition of cytochrome oxidase and blue-light damage in rat retina. Graefes Arch Clin Exp Ophthalmol 1993;231:416-23. [Crossref] [PubMed]
- Penn JS, Williams TP. Photostasis: regulation of daily photon-catch by rat retinas in response to various cyclic illuminances. Exp Eye Res 1986;43:915-28. [Crossref] [PubMed]
- Li J, Edward DP, Lam TT, et al. Amelioration of retinal photic injury by a combination of flunarizine and dimethylthiourea. Exp Eye Res 1993;56:71-8. [Crossref] [PubMed]
- Harada T, Harada C, Nakayama N, et al. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron 2000;26:533-41. [Crossref] [PubMed]
- Koyama Y, Kaidzu S, Kim YC, et al. Suppression of Light-Induced Retinal Degeneration by Quercetin via the AP-1 Pathway in Rats. Antioxidants (Basel) 2019; [Crossref] [PubMed]
- Chen P, Lai Z, Wu Y, et al. Retinal Neuron Is More Sensitive to Blue Light-Induced Damage than Glia Cell Due to DNA Double-Strand Breaks. Cells 2019; [Crossref] [PubMed]
- Hu S, Ma AD. LED lamps affect the elements of drawing among drawing personnel. Ju She 2019;8:167-8.
- Kremers JJ, van Norren D. Retinal damage in macaque after white light exposures lasting ten minutes to twelve hours. Invest Ophthalmol Vis Sci 1989;30:1032-40. [PubMed]
- Jaadane I, Boulenguez P, Chahory S, et al. Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic Biol Med 2015;84:373-84. [Crossref] [PubMed]
- Nakamura M, Kuse Y, Tsuruma K, et al. The involvement of the oxidative stress in murine blue led light-induced retinal damage model. Biol Pharm Bull 2017;40:1219-25. [Crossref] [PubMed]
Cite this article as: Wu G, Huang X, Meng H, Yang L, Lin S, Gao Y, Li Y, Wang Y. Retinal damage after exposure to white light emitting diode lights at different intensities in Sprague-Dawley rats. Ann Eye Sci 2019;4:24.


