
Femtosecond laser-assisted cataract surgery (FLACS) in resident training
Introduction
Cataracts are the leading cause of blindness, and cataract surgery is the most common ocular surgery performed worldwide with more than 20 million surgeries done annually (1). In the U.S., more than two million cataract surgeries are performed every year (2). Developing lens opacification is an age-related process; therefore, as life expectancy progressively increases, the number of cataract surgeries is expected to rise.
Brief history of cataract surgery
The procedure known as couching was first practiced in the 6th century B.C. in India (3). Couching involves a sharp instrument, which is used to penetrate the cornea or sclera and then dislocate the cataractous lens by pushing it into the posterior chamber of the eye. The patient subsequently uses aphakic correction in order to see. Couching was popular and spread to various ancient civilizations, including ancient Rome and Egypt, and was practiced for thousands of years. Centuries later, in 1747, Jacques Daviel of France performed the first extracapsular cataract extraction through an inferior corneal incision and a needle inserted behind the lens (4).
The most notable advances in cataract surgery have been made just in the past 100 years. In 1949, Sir Harold Ridley implanted the first intraocular lens (IOL) (4). Ridley was inspired by the observation of inert flecks of glass and plastic lodged inside the eyes of injured airmen in World War II. Then, in 1967, Dr. Charles Kelman invented the phacoemulsification probe after being inspired by a dentist visit in which he had a similar instrument used to clean his teeth (4). The following decades saw advances such as the invention of foldable IOLs, allowing insertion of an implant through a smaller incision, as well as multifocal and toric IOLs. In 2010, the U.S. Food and Drug Administration (FDA) approved the use of the femtosecond laser for cataract surgery.
Femtosecond laser assisted cataract surgery: development and significance
Femtosecond laser has a wavelength of 1,053 nm and a pulse duration in the femtosecond range (10-15 seconds). This short pulse duration reduces the amount of collateral tissue damage, compared to the slower excimer and neodymium-doped yttrium aluminum garnet (Nd:YAG) lasers (5). This allows the femtosecond laser to be used for procedures that require extreme precision. The femtosecond laser works on the principle of photodisruption: converting laser energy into mechanical energy. This is brought about by a tightly focused beam of ultrashort pulses of light energy with enough peak power to create plasma. This plasma free of electrons and ionized molecules rapidly expands, and cavitation bubbles that enlarge and coalesce are then created, being able to separate tissues (6-8). Compared to Nd:YAG laser, femtosecond is more innocuous due to smaller cavitation bubbles, in contrast to the ones produced by longer pulses used in Nd:YAG (9).
Femtosecond laser started being used in surgery in an effort to reduce collateral damage during procedures (10). Femtosecond laser’s first use in ophthalmology was for LASIK (laser-assisted in situ keratomileusis) surgery, a refractive surgery on the cornea in which the excimer laser and traditionally microkeratome were combined for the correction of myopia, hyperopia, and astigmatism. Femtosecond laser is a substitute for the microkeratome in the creation of the corneal flap in refractive surgery. In 2001, the first femtosecond laser (IntraLase, Johnson & Johnson Vision) received approval by the FDA. Advantages of using femtosecond laser over the microkeratome in LASIK are a considerable reduction in flap thickness, generation of a smoother cut compared to the microkeratome, and a more sterile procedure (10).
Femtosecond laser can also be used in keratoplasty to make the donor and recipient corneal cuts. The laser provides greater precision and smoothness of the cut, more host tissue conservation, and geometric cutting in an attempt to decrease wound leakage after keratoplasty (10). Femtosecond laser has also been used experimentally for correcting presbyopia through treatment of the stromal layer in an attempt to steepen the central portion of the anterior cornea (11). In keratoconus patients, the femtosecond laser can be used to provide the potential corneal tunnels into which intracorneal ring segments are placed (12).
In LASIK, bridging tissue remains after treatment, so the flap must be separated from the remainder of the cornea after femtosecond laser delivery. This is due to non-overlapping spots during performance of the laser. However, in FLACS, spot settings do overlap to create cavitation regions.
While femtosecond laser’s use in LASIK is well-established, the introduction of femtosecond laser to cataract surgery has posed more of a challenge. This is because only one interface, the cornea, needs to be considered in LASIK. In contrast, the FLACS procedure requires adaptation to three different interfaces with differing densities: cornea, anterior chamber containing aqueous humor, and lens (see Figure 1). Furthermore, in LASIK, the laser only needs to be focused on the corneal epithelium and stroma. In FLACS, the laser beam must be able to focus on different structures at different depths (i.e., cornea, anterior capsule, lens). Any interfering structure between the laser and the interface being treated, such as bubbles, corneal folds, or corneal scars, could result in a different outcome than expected, such as possible damage to the surrounding structures or no treatment delivered due to lack of penetration of the laser.
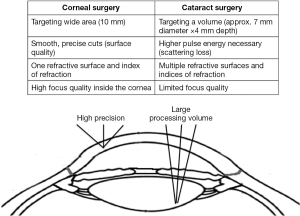
The aforementioned challenges were eventually overcome through years of research, and the femtosecond laser was successfully introduced into cataract surgery in 2009 and approved for use in cataract surgery by the FDA in 2010 (7,8,13-15). Dr. Zoltan Nagy of Semmelweis University in Budapest was the first to use femtosecond laser for cataract surgery in 2009. In FLACS, the surgeon can customize treatment by selecting which steps the laser will perform in each surgery. Different parameters can be applied for the various steps performed by the femtosecond laser: capsulorhexis, lens fragmentation, arcuate keratotomies, and corneal incisions.
The laser treatment is always preceded by a docking procedure, which connects the patient to the treatment interface. To avoid a significant deformation of the eye during the docking procedure, several types of interfaces are used by different laser platforms. The interfaces can be non-applanating or applanating. In the non-applanating system, a fluid interface can be used, such as in the Catalys (Johnson & Johnson Vision, Inc.) and LENSAR (LENSAR, Inc.) platforms. With the applanating system, a curved contact lens between the corneal surface and the rigid curved interface can be used, as in the LenSx (Alcon Laboratories, Inc.) platform, or a curved glass interface can be present, as in the Victus (Bausch + Lomb, Inc.) platform (5,8). The goal of the docking system is to achieve a stable and appropriate eye position to allow the cornea and other transparent tissues to undergo live imaging and precise laser beam delivery (16).
The Liquid Optic interface (LOI) used by the Catalys laser is a non-applanating system composed of different parts, including a cup that directly faces the eye that is filled with balanced salt solution (BSS). This cup also has a suction ring surrounding it, as well as a disposable window, which is immersed in the liquid, eliminating any gap between the cornea and the solution. First, the suction ring fixes the eye, then the cup is filled with BSS, and finally the disposable window is introduced and connected to the BSS filled cup. This setup is similar to the interfaces used for ultrasound in which the refractive effect can be minimized because cornea and balanced salt solution have very close indices of refraction (17). Schultz and colleagues (16) postulate that the LOI has an advantage over flat and curved interfaces because it does not cause as much of a rise in intraocular pressure and also produces fewer corneal folds while suctioning because the eye is less deformed. According to Talamo et al. (17), the LOI eliminates the possibility of corneal folds appearing, which could contribute to incomplete capsulotomies.
The first step performed in FLACS is the capsulorhexis. Anterior capsular incisions are considered one of the most challenging steps in cataract surgery by novice surgeons. The goal of femtosecond laser in this step is to achieve a more symmetric and reproducible capsulorhexis, which is of particular importance in cases where multifocal or toric IOLs are implanted, given the role of the capsulorhexis in determining effective lens position (ELP). Importance of the size of the capsulotomy is reflected in a probable hyperopic shift if the capsulotomy is too small or in myopic shift and IOL decentration when too large (9). In cases of loss of suction, this step should be completed with the cystotome. There are differing opinions regarding capsular strength when using femtosecond compared to conventional phacoemulsification. Palanker et al. (18) proposes that femtosecond seems to double capsulotomy strength and increase fivefold the accuracy of the size and shape of the rhexis compared to manual performance. However, Serrao et al. (19) found edge irregularities in capsulotomies performed by femtosecond laser that are not found in manual capsulotomies, which they propose decrease the stretchability of the capsule, leading to an increased risk of anterior capsular tears. Nonetheless, it is undeniable that the femtosecond laser increases the predictability and reproducibility of the capsulotomy in terms of size, centration, and circularity.
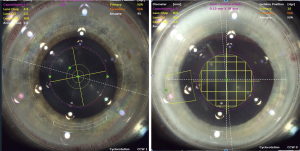
The next femtosecond laser step in cataract surgery is lens fragmentation. There are different patterns of lens fragmentation available, which can be customized depending on the type of cataract, the anatomic characteristics of the eye, or based on the surgeon’s preferences. Lens fragmentation patterns consist of multiple combinations of different numbers of cylinders, wedges, and grids. Figure 2 depicts different fragmentation patterns available on the LenSx platform. There are a limited number of published studies on the effect of different fragmentation patterns on surgical outcomes, but it can be postulated that different fragmentation patterns would be useful for certain types of cases. Conrad-Hengerer et al. (20) have shown that smaller fragmentation patterns (i.e., smaller grids) can lead to reduced effective phacoemulsification time (EPT). A group from Germany has shown that, for less dense lenses, grid fragmentation leads to reduced EPT compared to a pie-cut pattern, but for denser lenses, the pie-cut pattern led to lower EPT than grid fragmentation (21). They state that this is because the pie-cut pattern can help with fragmentation in denser lenses, likely due to assisting with lens cracking and chopping. Further studies are needed to elucidate the ideal type of fragmentation pattern for various types of cataracts. However, FLACS is a versatile tool because it can be adapted to the surgeon’s preference.
The femtosecond laser can be used to create the clear corneal incision (CCI) and paracentesis wounds in cataract surgery. It can be difficult to control the length and architecture of the incision when manually creating CCIs (22). The wounds created by the femtosecond laser are consistently triplanar and square, theoretically leading to a reduced risk of leakage and therefore reduced risk of resultant complications, including endophthalmitis. Masket et al. (23) showed that femtosecond laser-created corneal incisions in cadaveric eyes resisted deformation and leakage at various intraocular pressures. Grewal and Basti (22) found lower rates of endothelial gape, endothelial misalignment, and Descemet membrane detachments in the femtosecond laser CCI group versus the manual CCI group.
The last step in femtosecond laser assisted cataract surgery is the creation of partial thickness arcuate corneal incisions, or astigmatic keratotomy (AK). AK has been used to treat mild to moderate astigmatism (up to 1.5 D) from a variety of causes, such as after trauma or corneal transplantation and astigmatism at the time of cataract surgery (24). These incisions can be performed free-handed with a mechanical keratome or with the femtosecond laser. Limbal relaxing incisions (LRI) are a subset of AK but feature more peripherally placed incisions. Manual AKs and LRIs are made most commonly with a diamond knife at a preset depth (25). Overcorrection, undercorrection, and corneal perforation are complications that can occur from manually created incisions. Femtosecond laser-created arcuate incisions created with image guidance can reduce these aforementioned risks.
Femtosecond arcuate incisions can be created at different depths. Some surgeons prefer performing anterior penetrating while others prefer intrastromal incisions. Small arcuate incisions can also be used as toric markers. Manual opening of anterior penetrating stromal incisions can be done during the time of cataract surgery or up to one month after the surgery to increase their effect. Sometimes, they do not require opening in cases where minimal residual error remains. Default settings for arcuate incisions are 80% depth at the limbus, which are based on normograms for manual incisions (5). New nomograms specific for femtosecond laser-created arcuate incisions need to be established in order to optimize this procedure. Femtosecond laser allows these parameters to be customized in terms of arc length, orientation, and depth. This is a major step considering the previous difficulties in performing these parameters, eliminating the need for manual measurements and the concern of blade variability.
Although limited literature about the advantages of femtosecond versus manual keratotomies is available, it is reported that laser is more accurate than manual cutting by the surgeon (26). Hoffart et al. (27) suggests that femtosecond arcuate incisions provided a larger reduction in astigmatism than a mechanized keratome with less misalignment. Patients suffering from high astigmatism after penetrating keratoplasty can also benefit from femtosecond laser-assisted arcuate keratotomies, which has been proven to be safe and effective (28).
Currently, four main femtosecond laser-assisted cataract surgery platforms are available: LenSx, Catalys, LENSAR, and Victus. Table 1 compares the features of these various platforms.
Table 1
| Features | LenSx | Catalys | Victus | LENSAR |
|---|---|---|---|---|
| Company | Alcon | Johnson & Johnson | Bausch + Lomb | LENSAR |
| Steps of cataract surgery performed | Corneal and arcuate incisions, anterior capsulotomy, lens fragmentation | Corneal and arcuate incisions, anterior capsulotomy, lens fragmentation | Corneal and arcuate incisions, anterior capsulotomy, lens fragmentation | Corneal and arcuate incisions, anterior capsulotomy, lens fragmentation |
| Capable of creating corneal flaps? (e.g., LASIK) | Yes | No | Yes | No |
| Patient interface docking type | Applanating, Softfit curved contact lens interface | Non-applanating liquid interface | Dual mode: non-applanating liquid interface for cataract surgeries, applanating curved interface for corneal procedures | Non-applanating liquid interface |
| Imaging type | Spectral-domain OCT | Spectral-domain OCT | Swept-source OCT | 3D ray-tracing confocal structural illumination (Scheimpflug principle) |
| Bed type | Wheeled bed (hospital gurney) | Fixed bed | Fixed bed | Wheeled bed (hospital gurney) |
The combination of the femtosecond laser with real time anterior segment imaging has provided a new perspective of cataract surgery. Image-obtaining technology varies among the different femtosecond laser platforms. 3-D OCT is used by Catalys, LenSx, and Victus, and 3D ray-tracing confocal structural illumination (CSI), similar to the Scheimpflug imaging developed for corneal topography, is used by LENSAR (5). The images provide a three-dimensional map, which allows one to identify the anterior and posterior surfaces of the cornea, lens, and lens capsule and makes cataract surgery a more visual process. Figure 3 shows the variability in lens thickness and position captured by intra-operative OCT imaging.
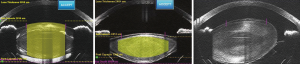
Imaging integration also lowers the probability of performing incisions where depth, location, and shape are inaccurate. Treatment patterns are determined using the OCT or ray-tracing CSI, which creates a 3-D map of the cornea, lens, and lens capsule, along with real time video. Axial cut imaging and live video are coupled in the same system, so the superimposed video allows the surgeon to review, verify, and modify the pattern of treatment according to the patient’s anatomy (18). A live video obtained through the same objective is projected with an overlying diagram of the capsulotomy circumference, lens fragmentation structure, incision location, and shape, allowing adjustment and verification of the parameters prior to execution of the laser (17).
The use of femtosecond laser-assisted cataract surgery has been well-studied. However, the role of FLACS in U.S. ophthalmology residency programs requires further investigation. There is a great deal of variability in the use of FLACS among different residency programs, from no exposure to actively performing all steps of the femtosecond procedure. The Accreditation Council for Graduate Medical Education (ACGME) requires ophthalmology residents to perform a minimum of 86 cataracts as primary surgeon in order to graduate. However, requirements are not well established for newer technologies, such as capsule stabilization tools, toric and multifocal IOLs, and femtosecond laser. Additionally, there is no universally required curriculum for teaching cataract surgery, which leads to a range of teaching methods and exposures to various types of technology, such as FLACS, intraoperative aberrometry, and multifocal IOLs (29).
Teaching cataract surgery to residents
Learning how to perform cataract surgery is an essential part of ophthalmology residency. Teaching cataract surgery to residents is a challenging task for various reasons (30,31). One reason is that microsurgery typically allows only one person to be performing the key operative steps at any given time. Current surgical simulators are unable to replace the experience of performing surgery on a live patient, so the novice surgeon goes into surgery performing techniques on a live patient that have not yet been mastered. Additionally, the delicate nature of the tissues involved in cataract surgery (e.g., posterior capsule that is less than five micrometers in thickness in areas) leads to a small margin of error, meaning that one misstep can lead to significant intraoperative repercussions (31). Lastly, the patient being awake and able to hear conversation during most cataract surgeries, which are typically performed under monitored anesthesia care, can limit the amount of timely and useful feedback from the supervising physician (30,31).
Unsurprisingly, multiple studies have shown that cataract surgeries performed early on by residents have a greater risk of vitreous loss compared to those performed later on in the learning curve (30,32).
As mentioned previously, there is no nationwide standardized curriculum for teaching cataract surgery, which leads to variability in how it is taught. Efforts have been made by various residency programs in an attempt to standardize the cataract surgery curriculum. For instance, the University of Iowa’s cataract surgical curriculum was implemented in 2004. This curriculum takes place during the first and second year of residency and includes structured wet lab and simulator training during the first year, assisting in the cases of senior residents during the first year, immediate feedback from faculty after performing certain parts of 10–20 cataract surgeries (with heavy faculty assistance during these early surgeries) during the second year of residency, and the practice of 50–80 capsulorhexis in live surgeries with a high volume surgeon during the second year (33).
Rogers et al. (33) showed that the rates of posterior capsular rupture and/or vitreous loss in resident-performed cataract surgery were significantly lower after implementation of the University of Iowa cataract surgical curriculum compared to rates before its initiation. The aforementioned group examined cataract surgeries performed by residents at their affiliated VA hospital five years before the curriculum (n=823) was enacted as well as four years after it was put into practice (n=1,009). The investigators controlled for surgical experience by grouping residents into cases performed early in the academic year versus later in the academic year. They found a statistically significant reduction in the rates of posterior capsular rupture and/or vitreous loss, from 7.17% before the curriculum change to 3.77% after the curriculum change. Similarly, Borboli-Gerogiannis et al. (34) at the Massachusetts Eye and Ear Infirmary showed that there was a reduced incidence of posterior capsular rupture and/or vitreous loss among resident cataract surgeries after the initiation of their own three-year cataract surgery curriculum, which included elements similar to that at the University of Iowa.
Educators have proposed various ways in which to introduce residents to the steps of cataract surgery. The University of Iowa advocates for a “backward” method, in which residents start by performing the last steps of surgery first and then ultimately move toward the capsulorhexis portion, which many see as one of the most challenging parts of cataract surgery. They promote this method because mistakes make in the later steps of surgery are less vision-threatening than those made in the earlier steps, such as during capsulorhexis formation or division of the nucleus (33). Similarly, University of Utah Moran Eye Center also employs this backward teaching technique (30). In addition to improving patient safety, educators state that this backward method of teaching also improves resident self-confidence by allowing novice surgeons to start performing steps on which they are more likely to succeed.
The ACGME requires ophthalmology residency programs to have either a wet lab or surgical simulator. Ophthalmologic wet labs often feature porcine eyeballs and the Kitaro cataract training system (Kitaro Dry Lab kits, FCI Ophthalmics Inc) for resident practice (34).
Staropoli et al. (35) at Bascom Palmer have shown that use of the Eyesi (VRmagic Holding AG) surgical simulator among their residents led to a 50% reduction in cataract surgery complication rates after it became mandatory in 2014 for its residents to use the simulator prior to performing live surgery. Roohipoor et al. (36) at Massachusetts Eye and Ear Infirmary have found that Eyesi scores could help with early identification of residents who need more help in learning cataract surgery. They showed that Eyesi training module scores early in residency correlate with later surgical performance.
FLACS in resident training
Yen and Ramanathan (29) surveyed ophthalmology residency program directors across the U.S. in 2016–2017 and found that, in 44% of programs, residents performed FLACS. In 25.4% of programs, they observed only; in 35.6%, they had didactic training only; and in 22%, no exposure was provided. An older survey performed in 2014 by Shah and Sullivan (37) reported that only 21% of U.S. residency programs were teaching FLACS to its residents. Therefore, based on those survey results, there has been more than a 20% increase in resident exposure to FLACS over 2–3 years.
Several studies have shown that FLACS does not pose any harm to patients when performed by residents and therefore can be safely integrated into the residency curriculum (38). For instance, Brunin and colleagues (39) at the Baylor College of Medicine performed a retrospective study comparing complication rates and refractive outcomes in manual cataract surgery versus FLACS in cases performed by third year resident surgeons. All surgeries were performed at their affiliated VA hospital. They found that complication rates were similar in the two groups, and visual outcomes were equal at both one month and one year after surgery. Cumulative Dissipated Energy (CDE) was notably lower in the FLACS group.
Pitter and Sullivan (40) from Loyola University Chicago published a similar retrospective study examining outcomes in resident-performed FLACS at their affiliated VA hospital. Their study also showed that complication rates were similar in FLACS and conventional surgeries. However, FLACS cases took longer than conventional surgery, with a mean difference in total operating time of 7.4 minutes. At this VA hospital, the femtosecond laser machine was located in the same room as the operating microscope, which is not the case at other hospitals and ambulatory surgical centers in which the laser is housed in a separate room. The authors of this study attributed the increased operating time in the FLACS group to the resident learning curve for FLACS.
Our group at the University of Illinois at Chicago published a study in 2015 that also showed that complication rates in FLACS were no higher than in manual cataract surgery when performed by residents and fellows (41). Additionally, phacoemulsification time, total operating room time (not including time for femtosecond laser use), cumulated dissipated energy, and irrigating fluid usage were lower in the FLACS group.
FLACS can be effectively incorporated into the stepwise teaching of cataract surgery. For instance, FLACS can be used to perform the most difficult steps of cataract surgery in inexperienced surgeons. This approach allows the novice to hone skills in the wet lab and on surgical simulators before embarking on those more challenging steps without the aid of the femtosecond laser (38). A case series by Taravella et al. (42) indicated that nucleus disassembly/removal and capsulorhexis were among the most challenging steps for trainee surgeons to master. FLACS facilitates completion of these more challenging steps of cataract surgery, allowing even the novice surgeon to perform a safe surgery in complex cases. Similarly, Cowan and Kloek (7) have suggested that FLACS could be a useful adjunct to teaching residents cataract surgery by allowing novice surgeons to bypass the more difficult steps of surgery early on in their training.
FLACS can play an important role in resident training when performing complex cataract surgeries, such as surgeries on brunescent cataracts, white cataracts, and cases with zonulopathy, which are situations in which experienced surgeons also find FLACS particularly useful. A study by Blomquist et al. (43) showed that risk factors for vitreous loss in resident-performed cataract surgery included all of the previously mentioned types of complex cataracts. Therefore, we propose that, if a resident is faced with a complex cataract early on in training, FLACS could be useful in helping teach a resident how to manage difficult cases intraoperatively while increasing the chances of a successful surgery.
A study performed at our institution showed that the complication rate for resident-performed complex cataract surgeries using the femtosecond laser was at the lower end of the reported complication rate for complex cataracts overall. The cataract complication rate in complex cases performed by experienced surgeons has been reported to range from 7–13% (44,45). In our study featuring 69 complex cataracts performed by residents and fellows using FLACS, the complication rate was 7.25% (n=4) (46). These complex cataracts included patients with pre-existing zonulopathy, Fuchs endothelial dystrophy, and hypermature cataracts. This study demonstrates that trainee-performed FLACS is safe in complex cataract surgery and does not lead to a complication rate higher than that of experienced surgeons.
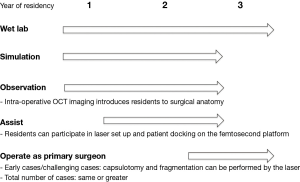
Some argue that FLACS introduces another element of surgery (e.g., docking the patient onto the laser platform, learning how to adjust laser settings) that has its own learning curve (47). Others also postulate that FLACS could cause loss of surgical skills among residents given that it automates several of the most challenging steps of cataract surgery (7). Regarding the latter point, FLACS must be taught in conjunction with conventional methods of cataract surgery as an adjunct rather than a replacement for budding surgeons. Figure 4 illustrates how the femtosecond laser can be integrated into the curriculum for teaching cataract surgery.
Conclusions
The role of FLACS in resident training continues to evolve, and current research suggests that the use of the femtosecond laser in cataract surgery is promising in terms of benefits for both the patient and the surgeon, particularly in cases of complex cataracts. Exposure to this technology in residency provides trainees with a well-rounded and modern surgical experience and prepares them for potential use of this technology in their careers as attendings. FLACS will likely become a more prominent player in residency programs in upcoming years, as more and more residency programs gain access to a femtosecond laser machine.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Joann Kang, Viral Juthani and Roy S. Chuck) for the series “Refractive Surgery” published in Annals of Eye Science. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2018.12.01). The series “Refractive Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. de la Cruz is a consultant for Alcon Laboratories, Inc., Fort Worth, TX. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lindstrom R. Thoughts on Cataract Surgery [Internet]. Review of Ophthalmology. 2015 [cited 20 Oct 2018]. Available online: https://www.reviewofophthalmology.com/article/thoughts-on--cataract-surgery-2015
- French DD, Margo CE, Behrens JJ, et al. Rates of Routine Cataract Surgery Among Medicare Beneficiaries. JAMA Ophthalmol 2017;135:163-5. [Crossref] [PubMed]
- Unite for Sight. Couching. Unite for Sight. [cited 20 Oct 2018]. Available online: http://www.uniteforsight.org/traditional-eye-practices/module3
- Jaffe NS. History of Cataract Surgery. Ophthalmology 1996;103:S5-16. [Crossref] [PubMed]
- Donaldson KE, Braga-Mele R, Cabot F, et al. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg 2013;39:1753-63. [Crossref] [PubMed]
- Sutton G, Bali SJ, Hodge C. Femtosecond cataract surgery: Transitioning to laser cataract. Curr Opin Ophthalmol 2013;24:3-8. [Crossref] [PubMed]
- Cowan LA, Kloek C. Introducing a New Surgical Technology- Controversies in Femtosecond Laser-assisted Cataract Surgery and Impact on Resident Surgical Training. Int Ophthalmol Clin 2015;55:23-35. [Crossref] [PubMed]
- Ali MH, Javaid M, Jamal S, et al. Femtosecond laser assisted cataract surgery, beginning of a new era in cataract surgery. Oman J Ophthalmol 2015;8:141-6. [Crossref] [PubMed]
- Hatch KM, Talamo JH. Laser-assisted cataract surgery: benefits and barriers. Curr Opin Ophthalmol 2014;25:54-61. [Crossref] [PubMed]
- Chung SH, Mazur E. Surgical applications of femtosecond lasers. J Biophotonics. 2009;2:557-72. [Crossref] [PubMed]
- Thomas BC, Fitting A, Khoramnia R, et al. Long-term outcomes of intrastromal femtosecond laser presbyopia correction: 3-year results. Br J Ophthalmol 2016;100:1536-41. [Crossref] [PubMed]
- Pajic B, Vastardis I, Gatzioufas Z, et al. First experience with the new high-frequency femtosecond laser system (LDV Z8) for cataract surgery. Clin Ophthalmol 2014;8:2485-89. [Crossref] [PubMed]
- Christy JS, Nath M, Mouttapa F, et al. Learning curve of femtosecond laser-assisted cataract surgery: Experience of surgeons new to femtosecond laser platform. Indian J Ophthalmol 2017;65:683-9. [Crossref] [PubMed]
- Nagy ZZ, Mastropasqua L, Knorz MC. The use of femtosecond laser in cataract surgery: Review of the published results within the LenSx system. J Refract Surg 2014;30:730-40. [Crossref] [PubMed]
- Ang RET, Quinto MMS, Cruz EM, et al. Comparison of clinical outcomes between femtosecond laser-assisted versus conventional phacoemulsification. Eye Vis (Lond) 2018;5:8. [Crossref] [PubMed]
- Schultz T, Conrad-Hengerer I, Hengerer FH, et al. Intraocular pressure variation during femtosecond laser–assisted cataract surgery using a fluid-filled interface. J Cataract Refract Surg 2013;39:22-7. [Crossref] [PubMed]
- Talamo JH, Gooding P, Angeley D, et al. Optical patient interface in femtosecond laser-assisted cataract surgery: contact corneal applanation versus liquid immersion. J Cataract Refract Surg 2013;39:501-10. [Crossref] [PubMed]
- Palanker DV, Blumenkranz MS, Andersen D, et al. Femtosecond laser-assisted cataract surgery with integrated optical coherence tomography. Sci Transl Med 2010;2:58ra85 [Crossref] [PubMed]
- Serrao S, Lombardo G, Desiderio G, et al. Analysis of femtosecond laser assisted capsulotomy cutting edges and manual capsulorhexis using environmental scanning electron microscopy. J Ophthalmol 2014;2014:520713 [Crossref] [PubMed]
- Conrad-Hengerer I, Hengerer FH, Schultz T, et al. Effect of femtosecond laser fragmentation of the nucleus with different softening grid sizes on effective phaco time in cataract surgery. J Cataract Refract Surg 2012;38:1888-94. [Crossref] [PubMed]
- Shajari M, Khalil S, Mayer WJ, et al. Comparison of 2 laser fragmentation patterns used in femtosecond laser-assisted cataract surgery. J Cataract Refract Surg 2017;43:1571-74. [Crossref] [PubMed]
- Grewal DS, Basti S. Comparison of morphologic features of clear corneal incisions created with a femtosecond laser or a keratome. J Cataract Refract Surg 2014;40:521-30. [Crossref] [PubMed]
- Masket S, Sarayba M, Ignacio T, et al. Femtosecond laser-assisted cataract incisions- architectural stability and reproducibility. J Cataract Refract Surg 2010;36:1048-49. [Crossref] [PubMed]
- Hays J. Astigmatic Keratotomy for the Correction of Astigmatism [Internet]. 2017 [cited 20 Oct 2018]. Available online: https://emedicine.medscape.com/article/1220380-overview#a6
- Lee BS, Lindstrom RL, Reeves SW, et al. Modern management of astigmatism. Int Ophthalmol Clin 2013;53:65-78. [Crossref] [PubMed]
- Chang JSM. Femtosecond laser-assisted astigmatic keratotomy: a review. Eye Vis (Lond) 2018;5:6. [Crossref] [PubMed]
- Hoffart L, Proust H, Matonti F, et al. Correction of postkeratoplasty astigmatism by femtosecond laser compared with mechanized astigmatic keratotomy. Am J Ophthalmol 2009;147:779-87. [Crossref] [PubMed]
- Vickers LA, Gupta PK. Femtosecond laser-assisted keratotomy. Curr Opin Ophthalmol 2016;27:277-84. [Crossref] [PubMed]
- Yen AJ, Ramanathan S. Advanced cataract learning experience in United States ophthalmology residency programs. J Cataract Refract Surg 2017;43:1350-5. [Crossref] [PubMed]
- Ament CS, Henderson BA. Optimizing resident education in cataract surgery. Curr Opin Ophthalmol 2011;22:64-7. [Crossref] [PubMed]
- Lee AG, Greenlee E, Oetting TA, et al. The Iowa ophthalmology wet laboratory curriculum for teaching and assessing cataract surgical competency. Ophthalmology 2007;114:e21-6. [Crossref] [PubMed]
- Randleman JB, Wolfe JD, Woodward M, et al. The resident surgeon phacoemulsification learning curve. Arch Ophthalmol 2007;125:1215-9. [Crossref] [PubMed]
- Rogers GM, Oetting TA, Lee AG, et al. Impact of a structured surgical curriculum on ophthalmic resident cataract surgery complication rates. J Cataract Refract Surg 2009;35:1956-60. [Crossref] [PubMed]
- Borboli-Gerogiannis S, Jeng-Miller KW, Koulisis N, et al. A Comprehensive Surgical Curriculum Reduced Intra-operative Complication Rates of Resident-performed Cataract Surgeries. J Surg Educ 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Staropoli PC, Gregori NZ, Junk AK, et al. Surgical Simulation Training Reduces Intraoperative Cataract Surgery Complications Among Residents. Simul Healthc. 2018;13:11-5. [PubMed]
- Roohipoor R, Yaseri M, Teymourpour A, et al. Early Performance on an Eye Surgery Simulator Predicts Subsequent Resident Surgical Performance. J Surg Educ 2017;74:1105-15. [Crossref] [PubMed]
- Shah RD, Sullivan BR. Resident training in femtosecond laser-assisted cataract surgery- national survey. J Cataract Refract Surg 2015;41:1531-3. [Crossref] [PubMed]
- Cohen MN, Intili A, Ni N, et al. Femtosecond laser-assisted cataract surgery in residency training. Curr Opin Ophthalmol 2015;26:56-60. [Crossref] [PubMed]
- Brunin G, Khan K, Biggerstaff KS, et al. Outcomes of femtosecond laser-assisted cataract surgery performed by surgeons-in-training. Graefes Arch Clin Exp Ophthalmol 2017;255:805-9. [Crossref] [PubMed]
- Pittner AC, Sullivan BR. Resident surgeon efficiency in femtosecond laser-assisted cataract surgery. Clin Ophthalmol 2017;11:291-7. [Crossref] [PubMed]
- Hou JH, Prickett AL, Cortina MS, et al. Safety of femtosecond laser-assisted cataract surgery performed by surgeons in training. J Refract Surg 2015;31:69-70. [Crossref] [PubMed]
- Taravella MJ, Davidson R, Erlanger M, et al. Characterizing the learning curve in phacoemulsification. J Cataract Refract Surg 2011;37:1069-75. [Crossref] [PubMed]
- Blomquist PH, Morales ME, Tong L, et al. Risk factors for vitreous complications in resident-performed phacoemulsification surgery. J Cataract Refract Surg 2012;38:208-14. [Crossref] [PubMed]
- Avramides S, Traianidis P, Sakkias G. Cataract surgery and lens implantation in eyes with exfoliation syndrome. J Cataract Refract Surg 1997;23:583-7. [Crossref] [PubMed]
- Erkayhan GE, Dogan S. Cataract Surgery and Possible Complications in Patients with Pseudoexfoliation Syndrome. Eurasian J Med 2017;49:22-5. [Crossref] [PubMed]
- Hassanaly S, Arteaga AC, and de la Cruz J. Maximizing outcomes of femtosecond laser-assisted cataract surgery in white and brunescent cataracts at an academic institution. Paper presented at the meeting of the American Society of Cataract and Refractive Surgery; USA; 2018 April 13-17.
- Kaplowitz K, Yazdanie M, Abazari A. A review of teaching methods and outcomes of resident phacoemulsification. Surv Ophthalmol 2018;63:257-67. [Crossref] [PubMed]
Cite this article as: Park J, Bueno CS, de la Cruz J. Femtosecond laser-assisted cataract surgery (FLACS) in resident training. Ann Eye Sci 2018;3:60.


