
急性视网膜动脉缺血
引言
急性视网膜动脉缺血包括血管性单眼一过性视力丧失(transient monocular vision loss,TMVL或短暂性视网膜缺血发作)、视网膜分支动脉阻塞(branch retinal artery occulsion,BRAO)、视网膜中央动脉阻塞(central retinal artery occlusion,CRAO)和眼动脉阻塞(ophthalmic artery occulsion,OAO),是急性单眼无痛性视力丧失的常见原因。任何阻断视网膜中央动脉(central retinal artery,CRA)血流的疾病均可引起急性视网膜动脉缺血。CRA由眼动脉(颈内动脉的第一个颅内分支)发出,主要供应包含黄斑部和中心凹在内的视网膜内层。
TMVL由CRA或其分支的一过性阻塞导致,引起的单眼视力丧失通常持续数分钟,随后患者视力可自行恢复,且无法检测到永久性视功能障碍[1-3]。而BRAO和CRAO由CRA或其分支发生较长时间的部分或完全阻塞引起,可导致永久性视功能障碍[视力下降和(或)视野缺损[4-6]。CRAO通常引起严重视功能障碍[视力极差和(或)视野严重缺损,而BRAO引起的视功能障碍相对较轻[4,7]。
睫状视网膜动脉发自睫状后循环而非CRA,15%~30%的人存在此动脉。因此,CRAO患者如果存在睫状视网膜动脉供应部分黄斑部和中心凹,其中心视力可接近正常(20/50及以上)[8-10],但患眼周边视力严重受损[9,11]。
急性视网膜缺血的发生率
TMVL是急性视网膜动脉缺血最常见的表现,人群中TMVL每年的发生率约为1.4‱[12,13],而CRAO每年的发生率约为0.1‱~0.2‱。急性视网膜动脉缺血的发生率随年龄增长而增加,可能是由于年龄增长,心血管疾病的患病率也随之增加。CRAO在80岁以上患者中的发生率约为1‱[14,15],约占眼科门诊患者的1‱[14,16,17]。
病因
急性视网膜缺血可大致分为动脉炎性(血管炎所致)和非动脉炎性(非血管炎所致)。除了本节段落简要讨论巨细胞动脉炎(giant cell ateritis,GCA)(又称颞动脉炎),术语TMVL、OAO、CRAO和BRAO均指非动脉炎性急性视网膜动脉缺血。
急性视网膜动脉缺血最常见由远处形成的栓子引起,与前循环脑梗死类似。视网膜栓子最主要来源于同侧颈动脉,其次为主动脉弓和心脏,而高凝状态、血管炎(如GCA)、某些眼部及系统性疾病形成的栓子较少见。
同侧颈内动脉粥样硬化狭窄也是急性视网膜动脉缺血最常见的原因。任何患者(尤其年轻患者)出现CRAO伴面/颈部疼痛或头痛时,应着重考虑是否存在同侧颈动脉夹层[18,19]。有研究表明霍纳综合征常与CRAO出现在同侧[19]。颈动脉夹层可自发出现,也可由颈部外伤或颈部脊椎按摩突然诱发。因此,必须详细询问病史,确定患者视力丧失前是否有颈部外伤或颈部按摩操作,以及患者是否有纤维肌发育不良(fibromuscular dysplasia,FMD)等可能导致动脉夹层的血管性疾病家族史[20]。虽然所有CRAO患者均应行颈动脉和主动脉弓血管成像,但对任何怀疑颈动脉夹层或有颈动脉夹层发生风险的患者应急行计算机体层血管造影(computer tomography angiogram,CTA)或磁共振血管造影(magnetic resonance angiogram,MRA)以评估颈动脉颅内外走行的情况。
虽然高凝状态或血管炎(如GCA)引起CRA或其分支形成血栓而导致急性视网膜缺血并不常见,但仔细检查仍未找到栓子来源时需考虑这些情况[9,21-26]。实际上,50岁以上的急性视网膜缺血患者如伴有颞侧头痛、颞动脉触诊压痛或下颌痛等系统症状,需考虑是否为GCA。GCA相关检查包括红细胞沉降率(erythrocyte sedimentation rate,ESR)、血小板计数和C反应蛋白(C-reactive protein,CRP)等炎症指标。如果实验室检查结果不明确但临床高度怀疑GCA时,为防止患者出现进一步视力丧失和系统并发症,应行颞动脉活体组织检查[8,9],同时大剂量静脉注射糖皮质激素,后改为口服强的松(起始量1 mg/kg)并缓慢减量[27,28]。
此外,某些眼部疾病(如某些青光眼患者出现眼压急剧升高)同样与CRAO发病有关[29,30]。俯卧位脊柱手术对眼部的压迫也可导致患者突发急性视网膜动脉缺血[29,30]。极少数情况下,牙齿或面部美容手术不慎将药物或充填材料注入面部血管也可导致急性视网膜动脉缺血发生[33]。
危险因素
急性视网膜动脉缺血的危险因素和病因与缺血性脑血管疾病(如高血压、动脉粥样硬化、糖尿病)的危险因素和病因相似[8,15,24,34-37]。欧洲眼内溶栓评估小组(the European Assessment Group for Lysis in the Eye,EAGLE)发起了一项评估CRAO动脉内溶栓疗效的大型多中心临床试验,共纳入77名CRAO患者。在这77名患者中,56名患者(73%)有高血压,31名患者(40%)颈动脉狭窄程度≥70%(大多数患者为单侧颈动脉狭窄),17名患者(22%)有冠心病,15名患者(19%)有心房颤动(atrial fibrillation,AF),13名患者(17%)有心脏瓣膜疾病。尽管大多数患者本身患有已知的心血管疾病,但是78%的患者发生CRAO时至少增加1个新的心血管危险因素[34]。
由于视网膜动脉缺血和脑缺血的危险因素和病因相似,美国心脏学会(American Heart Association,AHA)和美国卒中学会(American Stroke Association,ASA)认为急性视网膜缺血等同于急性脑缺血。在2013年共识声明中,AHA和ASA将中枢神经系统梗死(卒中)定义为“基于永久性损伤的神经病理学、神经影像学和(或)临床证据,由缺血导致的脑、脊髓或视网膜细胞死亡”[38]。因此,TMVL被认为是发生在视网膜的短暂性脑缺血发作(transient ischemic attack,TIA),而BRAO、CRAO和OAO被认为是发生在视网膜的脑缺血(卒中)[37,38]。
急性视网膜缺血的诊断
急性视网膜动脉缺血通常表现为患眼突发无痛性视力丧失和(或)视野缺损。研究表明TMVL可出现一系列视力障碍,即从视物“变灰”或“变暗”到视力完全丧失[12,39]。TMVL通常持续数分钟,但很少持续甚至超过1个小时[12,40]。由于视力丧失发作后可恢复正常,大多数TMVL患者的检查结果无明显异常。因此,需根据患者详细的病史诊断TMVL和找出潜在病因[12,39,41]。
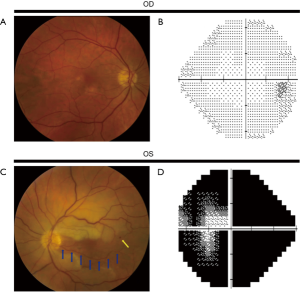
相较于TMVL,BRAO和CRAO可导致永久性视功能障碍[视力丧失和(或)视野缺损]。BRAO或CRAO患者的视力可接近正常,也可下降至数指(counting fingers,CF)甚至更差[4,7,42]。在一项纳入260只CRAO患眼的研究中[6],74%的患眼视力为CF及以下,视力较好者大多有一支睫状视网膜动脉供应部分或整个中心凹。患眼色觉减退与视力下降程度相称,且患眼可出现相对性传入性瞳孔障碍(relative afferent pupillatry defect,RAPD)。散瞳眼底检查可见患眼视网膜缺血区水肿(视网膜明显变白)(图1,图2)、CRAO樱桃红斑(中心凹神经纤维层很薄而其下脉络膜循环正常,故中心凹呈粉红色或红色)、视网膜小动脉血流缓慢呈节段状(类似“火车车厢”)、视网膜小动脉变细而视神经正常[8,43],有时可见CRA或其分支的栓子。眼动脉阻塞无樱桃红斑,但会出现视盘水肿。急性期患眼视网膜表现可能不存在或不明显,但视网膜表现可能在数小时后出现[8,25,43]。当预期的检查结果不明显或为阴性,以及患者诊断不明确时,可通过光学相干断层扫描(optical coherence tomography,OCT)、OCT血管成像(OCT angiography,OCTA)或荧光素血管造影(fluorescein angiogram,FA)协助诊断视网膜动脉阻塞(CRAO或BRAO)。FA(图2)和OCTA可见视网膜小动脉充盈延迟或无灌注[25,44]。OCT可见CRA供血不足造成的视网膜损伤,急性期表现为视网膜水肿,后期表现为视网膜内层变薄或破坏[45]。
发病率和死亡率
视力预后
OAO和CRAO通常引起严重视力丧失和(或)视野缺损,而BRAO引起的视功能障碍通常较CRAO轻[4,6,7,42]。BRAO和CRAO的视力预后不定,具体取决于CRA或其分支阻塞的时间、栓子类型等多种因素,CRAO的视力预后还与是否存在睫状视网膜动脉有关[11]。一项纳入244名CRAO患者的研究表明,无睫状视网膜动脉的患者中,93.2%的患者初诊视力≤CF;有睫状视网膜动脉的患者中,60%的患者初诊视力≤CF且病变未累及中心凹。无睫状视网膜动脉的患者中,仅有一位患者初诊视力≥20/40;有睫状视网膜动脉的患者中,20%的患者初诊视力≥20/40。初诊视力≤CF但视力部分恢复的患者比例在有睫状视网膜动脉的患者中为47%,无睫状视网膜动脉的患者中为16%。最终视力较初诊视力更差的患者比例在有睫状视网膜动脉的患者中为6%,无睫状视网膜动脉的患者中为8%[6,11]。无论是否存在睫状视网膜动脉,视野检查显示中心暗点的患者中约有20%~25%的患者中心暗点得到改善[6]。尽管研究表明存在睫状视网膜动脉的患者视力恢复的可能性大,但是大多数CRAO患者视力不会自行恢复且患眼持续存在严重视功能障碍。
BRAO患者的视力通常较CRAO好,据报道BRAO患者的视力至少为CF[4,7,42]。一项纳入212只BRAO眼的研究发现,非动脉炎性BRAO患者中79.5%(124/156)的患者初诊视力≥20/40[42]。BRAO患者不仅初诊视力较好,而且视力损害和视野缺损恢复的可能性更大,大多数BRAO患者的视力可以保持或恢复到20/40及以上[4,7,42]。
影响患者最终视力的最主要决定因素很可能是CRA或其分支阻塞的时间。在CRAO灵长类动物(非人类)模型中,CRA阻塞100分钟内未能检测到视网膜损伤。但CRA阻塞100到240分钟可造成不同程度的永久性视网膜损伤,且CRA阻塞约240分钟后可导致视网膜产生严重且不可逆的损伤[5,46]。这些研究表明,急性视网膜动脉缺血发生后,患者能否恢复视功能与视网膜缺血持续时间有关。因此,与急性脑梗死相似,急性视网膜动脉缺血很可能也存在一个独立的时间窗,在此时间窗内恢复视网膜血流可能有助于提高患者的视力预后。理论上患者诊断越迅速、视网膜血流重建越及时,视力越有可能恢复[5,11,46]。
眼部并发症
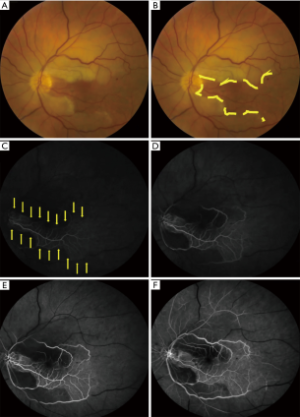
眼部新生血管是急性视网膜动脉缺血已知的并发症,最早可形成于急性视网膜动脉缺血发病2周后,可进一步导致患者视力严重丧失[47]。CRAO和BRAO均可导致眼部新生血管产生,但CRAO更为常见[48,49]。眼前段新生血管可导致新生血管性青光眼,引起眼痛、眼压明显升高和视力急剧下降;视网膜新生血管可导致玻璃体积血和(或)牵拉性视网膜脱离。因此,为防止患者视力进一步丧失,一旦发现新生血管应转诊给玻璃体视网膜疾病专科医生。
与系统性疾病的关系
由于急性视网膜缺血可导致永久性视力严重丧失,引起早期和晚期眼部及系统并发症,故CRAO甚至BRAO的发病率和死亡率均较高[35,50-54]。CRAO和BRAO通常单眼发病,引起严重肢体残疾的可能性小于脑缺血[55]。然而CRAO和BRAO均可导致严重视力下降和(或)视野缺损,进而造成患者的生活质量降低和独立生活能力下降,甚至需要机构照顾[50]。由于存在视功能障碍,急性视网膜动脉缺血造成视力严重丧失的患者跌倒和继发髋骨骨折的风险也很高,因而可能进一步降低患者的生活质量和独立生活能力。
除了视力损害,急性视网膜缺血还与较高的系统性疾病发病率有关。急性视网膜动脉缺血患者发病前有近期非眼部缺血性疾病[脑梗死或心肌梗死(myocardial infarction,MI)]发生史的可能性较大,发病后随之发生脑梗死或MI的风险也较高。急性视网膜缺血患者发病后1周内发生脑梗死或MI的风险最高[24,30,34-36,53,54,56,57],且CRAO或BRAO患者发生脑梗死或MI的风险在发病后长达10年内仍高于正常人群[54,48]。一项关于台湾人群的研究显示,CRAO患者发病后3年内发生卒中的风险是对照组的2.7倍,且CRAO患者发病后第1个月内卒中的发生率最高[58]。在EAGLE发起的临床试验中,77名CRAO患者中有5名在CRAO发病后第1个月内发生卒中,且这5名患者有4名于CRAO患眼同侧存在重度颈动脉狭窄[34]。另有研究表明,CRAO患者发病后第1年内卒中发生率高达13%,且CRAO患者发病后3.5年内卒中的发生率可能较正常人群高10倍。
CRAO患者除了卒中发生的风险增加,其心血管疾病(血管性死亡或MI)的死亡率也高于正常人群[34,51,53]。
综上,急性视网膜动脉缺血可能是患者有潜在系统性疾病的征兆,此类患者随后发生心血管缺血或脑缺血的风险高于正常人群,容易造成患者生活质量降低、独立生活能力下降,进而导致死亡率增高。
管理
TMVL被认为是发生在眼部的TIA,TMVL患者的急性期处理与永久性视网膜缺血患者类似[59]。因此,本综述未单独讨论TMVL的具体处理措施,TMVL患者的处理应根据永久性视网膜缺血性疾病(BRAO和CRAO)的管理指南。
AHA,ASA和多家国际卒中机构将CRAO和BRAO同卒中归为一类,急性期CRAO或BRAO患者需进行与急性脑缺血患者类似的评估。因此,急性视网膜缺血是眼科医疗急症,此类患者需立即转诊到距离最近的认证卒中中心进行详细评估,以明确急性视网膜缺血的病因/来源,并对随后可能出现的缺血并发症(MI或脑梗死)采取二级预防措施[6,22,53,54,57,59,60]。
近来多项研究表明,尽管就诊时缺乏提示急性脑缺血的其他局灶性神经功能障碍的表现,磁共振弥散加权成像(diffusion weighted imaging- magnetic resonance imaging,DWI-MRI)结果显示急性视网膜缺血患者中约有15-25%的患者发病时并发小范围的急性脑梗死[8,21,37,52,59,61-64]。无症状性脑梗死患者未来发生卒中的风险很高,但大多数急性无症状性脑梗死和急性视网膜缺血的患者常可找到主要病因,故此类患者常需抢救性治疗以预防之后发生卒中[52,61,63,64]。因此,即使缺乏其他局灶性神经功能障碍的表现,急性视网膜缺血患者也需急行DWI-MRI以判断是否并发脑缺血。
由于急性视网膜缺血最常见的原因是栓子阻塞了CRA或其分支,故急性视网膜缺血患者应重点检查是否存在形成栓子的潜在病因。应借助当地可用的医疗资源,为急性视网膜缺血患者急行颈动脉和主动脉弓血管成像、头颈部MRA、脑DWI-MRI或CTA。此外,所有急性视网膜缺血患者应行心脏评估,具体包括血压监测、心电图(electrokardiogram,EKG)、超声心动图(最好是气泡对比的经食管超声心动图检查)和心脏监护(患者在卒中中心检查超过24小时的情况下可替代动态心电图监护)[34]。
未明确栓子来源的年轻患者需行进一步检查,具体包括高凝状态(如凝血因子Ⅴ缺乏、蛋白C和蛋白S缺乏、高同型半胱氨酸血症、抗磷脂抗体、血小板增多、凝血酶原基因突变、抗凝血酶缺乏、高黏滞综合征)、风湿性系统性炎症疾病和血管阻塞性疾病(如镰状细胞病)的相关检查,及是否存在非法药物(静脉注射毒品和可卡因)和特定药物(鼻血管收缩药)的使用[29,65]。
目前处理方案
尽管AHA,ASA和多家国际卒中机构强烈建议急性视网膜缺血患者应在认证的卒中中心进行紧急评估,但许多眼科医生及神经科医生并未将急性视网膜缺血患者适当转诊[59,66-68]。2009年对美国乔治亚州眼科医生的调查显示,仅有约35%的眼科医生能将急性CRAO患者转诊到急诊科行进一步检查和危险分层[67]。2017年对美国玻璃体视网膜疾病专科医生和神经科医生的调查显示, 仅18%的玻璃体视网膜疾病专科医生及75%的神经科医生建议一名52岁、发病不足12小时的急性栓塞性视网膜动脉阻塞患者转诊至卒中单元或急诊室(emergency room,ER)[66]。此项研究还表明,仅8%的玻璃体视网膜疾病专科医生和46%的神经科医生建议发病24-48小时的急性视网膜动脉阻塞患者住院检查[66]。
急性视网膜动脉阻塞患者的紧急评估十分必要,需仔细检查以找出急性视网膜缺血的病因,如为同侧重度颈动脉狭窄、心房颤动等应紧急处理;同时采取二级预防措施,最大限度降低随后发生眼部、心血管或脑缺血性疾病的可能[60]。急性视网膜动脉缺血患者应在设有认证卒中中心的急救中心进行紧急评估,以便卒中神经科医生能对患者进行迅速、有效的会诊和检查。检查通常在24小时内进行,根据最初的检查结果确定患者是否需住入卒中病房紧急处理主要病因;或采取二级预防,卒中神经科医生适当随访。
急性视网膜缺血(CRAO或BRAO)的治疗
CRAO或BRAO的治疗可分为两部分:一是提高BRAO/CRAO患者视力预后的抢救性治疗;二是对随后可能发生的缺血性事件进行二级预防。尽管许多治疗措施曾用于尝试恢复BRAO/CRAO患者的眼部血流灌注和提高视力预后[3,8,9,22,25,69-71],但目前仍无治疗措施能够基于BRAO/CRAO的疾病自然史提高患者的视力预后[3,71]。理论上说,视网膜缺血持续的时间越短,视功能恢复的可能性越大(即越早恢复视网膜血流,患者的视功能越有可能恢复)[5,6,46,72]。灵长类动物急性视网膜缺血的研究表明,与急性脑缺血的推荐处理方案类似,在动物视力丧失3小时内给予治疗有助于预防永久性视网膜缺血。视力丧失6到12小时内治疗也可能有助于恢复视功能,但若视力丧失12小时及以上再行治疗则难以恢复视功能。许多研究中的治疗措施常在患者视力丧失>12小时后实施,故这些研究的价值有限。综上,在发现有治疗措施能使患者视功能改善程度超过急性视网膜缺血自然史预期前,患者的治疗应侧重于对随后可能发生的系统性缺血事件的二级预防。
保守治疗
许多BRAO或CRAO的保守疗法(也称“经典”或“传统”疗法)旨在恢复视网膜血流及改善视功能,具体包括机械清除栓子、升高视网膜动脉灌注压和增加血氧分压。
机械清除栓子的方法包括两种,一种是眼球按摩,通过扩张视网膜小动脉及降低眼压(intraocular pressure,IOP)来升高视网膜动脉灌注压[17,22,73];另一种是用钕钇铝石榴石(Neodymium: yttrium aluminum garnet,Nd: YAG)激光机械清除可见栓子[74,75]。研究表明,无论单用眼球按摩还是眼球按摩联合降眼压药物,都未能明显改善急性视网膜缺血患者的视功能[17,22,76]。Nd:YAG激光机械溶栓法可引起严重的副作用,如玻璃体积血和CRA假性动脉瘤形成[74,75],故该方法存在争议,尚不能作为急性视网膜缺血的标准治疗方案。
除了眼球按摩,研究者们也尝试过其他增加视网膜动脉灌注的方法,如应用降眼压药物[17,77]和前房穿刺术[44,78]。然而,这些治疗方法同样未能明显改善急性视网膜缺血患者的视功能[17,69,76,78,79]。
此外,研究者们还尝试过一些能扩张视网膜小动脉并减轻氧诱导的视网膜血管收缩的方法来改善急性视网膜缺血患者的视功能,如过度通气、吸入混合氧(95%氧及5%二氧化碳混合气体)和使用某些药物(硝酸异山梨酯和己酮可可碱)[17,78,80-84]。然而目前尚无文献依据表明这些疗法能够提高CRAO患者的视力预后[78]。
高压氧可增加血液中可溶性氧的浓度,曾被建议用于治疗急性视网膜缺血[85,86]。但同上述各种疗法类似,目前尚无文献依据支持高压氧能使患者视力预后的改善程度超过急性视网膜缺血自然史预期[87,88]。
溶栓治疗
溶血栓药包括尿激酶、链激酶和组织型纤溶酶原激活物(tissue plasminogen activator,tPA)等,能将纤溶酶原转化为纤溶酶,溶解纤维蛋白性血栓[22,24,25]。由于急性视网膜缺血最常见的血栓类型是纤维蛋白性血栓,且溶血栓药已有效用于急性脑缺血的治疗,故溶血栓药也可用于治疗CRAO。目前急性视网膜缺血溶血栓药的使用尚无标准治疗方案,大多数临床医生是根据已有的卒中治疗方案来决定是否对急性视网膜缺血患者使用溶血栓药。有研究表明溶血栓药未能明显改善急性视网膜缺血患者的视功能[89,90],一些回顾性综述和观察性研究也表明tPA不能明显改善视功能[91-93];但是也存在一些观察性研究、病例报道和回顾性综述认为tPA能够改善视功能[94-101]。大多数研究中患者视力丧失距离使用溶血栓药的时间>12小时,故患者视力改善不佳可能与患者视力症状出现距离使用溶血栓药的时间有关[72,96,101-105]。最近有研究分析了5项关于尿激酶(45名患者)或tPA(73名患者)治疗CRAO的回顾性病例系列研究,未发现患者视力症状出现距离溶栓治疗的时间与视功能改善之间的相关性[106]。另有研究表明,溶血栓药不仅未能有效改善急性视网膜动脉缺血患者的视力,而且易产生并发症[89]。因此,临床上应仔细权衡溶栓治疗的利弊,根据急性视网膜缺血患者的具体情况决定是否使用溶血栓药。
EAGLE发起的临床试验[107]对比了动脉内注入tPA与保守治疗(每日口服阿司匹林、血液稀释、应用降眼压药、眼球按摩和静脉注射肝素)对急性期CRAO患者(视力丧失<20小时)的疗效,其中42名患者(51.2%)局部眼动脉内注入tPA。结果显示,tPA溶栓组57%的患者和保守治疗组60%的患者视力提高≥3行,tPA溶栓组患者视力提高程度与保守治疗组相比无统计学差异。此外,tPA溶栓组37.1%的患者和保守治疗组仅4.3%的患者出现头痛、轻偏瘫、术后出血、颅内出血、鼻出血、口腔出血等,说明溶栓组较保守治疗组更易引起不良反应。由于tPA溶栓组患者视功能无明显改善且不良事件发生率高,故此项研究在第一个中期分析时便过早停止[107]。
根据目前已有的文献,尚不能总结出急性视网膜缺血的溶血栓药使用指南,主要原因有:研究设计(不同的药物治疗方案、急性视网膜缺血发病后不同的给药时间)及研究终点的异质性;一些随机对照试验结果显示使用tPA不能改善视功能,而一些回顾性研究和观察性研究表明使用tPA可能有助于提高视力预后;有研究显示使用溶血栓药会增加不良事件发生的风险[102]。因此,溶栓疗法的最佳给药时机和给药剂量,以及早期溶栓是否较保守疗法更能显著改善患者的视功能,这仍需进一步研究[108]。此外,设计与实施此类研究还需克服急性视网膜缺血相对罕见、患者治疗不及时等一系列困难[59,68,109-111]。
综上所述,急性视网膜缺血相当于急性脑缺血(TMVL相当于TIA,BRAO/CRAO相当于卒中),属于眼科医疗急症。由于目前缺乏可靠文献证实保守治疗或溶栓治疗改善视功能的有效性,急性视网膜缺血患者的处理应侧重于确定病因、治疗任何需紧急处理的病因、识别并发的急性脑梗死,并尝试通过减轻已知心血管危险因素的影响及识别新的心血管危险因素来预防随后可能发生的缺血事件(急性MI、血管性死亡和脑梗死)。急性视力丧失的患者最初可能就诊于眼科(多与眼科医生或验光师有关),一旦患者诊断为急性视网膜缺血,应立即转诊至距离最近、设有认证卒中中心的急救中心。急性视网膜缺血患者的最佳处理方案需要卒中神经科医生和眼科医生的合作,卒中神经科医生应急行相关检查,减轻已知心血管危险因素的影响,找出随后可能发生系统性缺血事件的具体病因;眼科医生应检查患者是否存在能导致患者视力进一步损害、丧失独立生活能力的眼部并发症(如继发新生血管),监测随后可能发生的视网膜缺血事件。
Acknowledgments
Funding: V Biousse and NJ Newman are supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness Inc., New York, by NIH/NEI core grant P30-EY006360 (Department of Ophthalmology, Emory University School of Medicine), and by NIH/NINDS (RO1NSO89694). M Dattilo is supported by the NEI sponsored teaching grant T32-EY007092 (Department of Ophthalmology). V Biousse received research support form NIH/PHS (UL1-RR025008). NJ Newman is a recipient of the Research to Prevent Blindness Lew R. Wasserman Merit Award.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Karl C. Golnik and Andrew G. Lee) for the series “Neuro-ophthalmology” published in Annals of Eye Science. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2018.05.04). The series “Neuro-ophthalmology” was commissioned by the editorial office without any funding or sponsorship. V Biousse and NJ Newman are consultants for GenSight Biologics. NJ Newman is a consultant for Santhera Pharmaceuticals. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marshall J, Meadows S. The natural history of amaurosis fugax. Brain 1968;91:419-34. [Crossref] [PubMed]
- Tippin J, Corbett JJ, Kerber RE, et al. Amaurosis fugax and ocular infarction in adolescents and young adults. Ann Neurol 1989;26:69-77. [Crossref] [PubMed]
- Vodopivec I, Cestari DM, Rizzo JF 3rd. Management of Transient Monocular Vision Loss and Retinal Artery Occlusions. Semin Ophthalmol 2017;32:125-33. [Crossref] [PubMed]
- Mason JO 3rd, Shah AA, Vail RS, et al. Branch retinal artery occlusion: visual prognosis. Am J Ophthalmol 2008;146:455-7. [Crossref] [PubMed]
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. Retinal survival time. Exp Eye Res 2004;78:723-36. [Crossref] [PubMed]
- Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol 2005;140:376-91. [Crossref] [PubMed]
- Yuzurihara D, Iijima H. Visual outcome in central retinal and branch retinal artery occlusion. Jpn J Ophthalmol 2004;48:490-2. [Crossref] [PubMed]
- Biousse V, Newman N. Retinal and optic nerve ischemia. Continuum (Minneap Minn) 2014;20:838-56. [Crossref] [PubMed]
- Cugati S, Varma DD, Chen CS, et al. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol 2013;15:63-77. [Crossref] [PubMed]
- Lorentzen SE. Incidence of cilioretinal arteries. Acta Ophthalmol (Copenh) 1970;48:518-24. [Crossref] [PubMed]
- Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res 2014;41:1-25. [Crossref] [PubMed]
- Lawlor M, Perry R, Hunt BJ, et al. Strokes and vision: The management of ischemic arterial disease affecting the retina and occipital lobe. Surv Ophthalmol 2015;60:296-309. [Crossref] [PubMed]
- Andersen CU, Marquardsen J, Mikkelsen B, et al. Amaurosis fugax in a Danish community: a prospective study. Stroke 1988;19:196-9. [Crossref] [PubMed]
- Park SJ, Choi NK, Seo KH, et al. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology 2014;121:1933-8. [Crossref] [PubMed]
- Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology 2009;116:1928-36. [Crossref] [PubMed]
- Leavitt JA, Larson TA, Hodge DO, et al. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol 2011;152:820-3 e2.
- Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol 1999;128:733-8. [Crossref] [PubMed]
- Patel M, Shah G, Davies JB, et al. Re-evaluating our perspective on retinal artery occlusion from carotid dissection: a report of three cases and review of the literature. Ophthalmic Surg Lasers Imaging Retina 2013;44:555-60. [Crossref] [PubMed]
- Biousse V, Touboul PJ, D'Anglejan-Chatillon J, et al. Ophthalmologic manifestations of internal carotid artery dissection. Am J Ophthalmol 1998;126:565-77. [Crossref] [PubMed]
- Narula N, Kadian-Dodov D, Olin JW. Fibromuscular Dysplasia: Contemporary Concepts and Future Directions. Prog Cardiovasc Dis 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Schmidt D, Hetzel A, Geibel-Zehender A, et al. Systemic diseases in non-inflammatory branch and central retinal artery occlusion--an overview of 416 patients. Eur J Med Res 2007;12:595-603. [PubMed]
- Chen CS, Lee AW. Management of acute central retinal artery occlusion. Nat Clin Pract Neurol 2008;4:376-83. [Crossref] [PubMed]
- Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res 2011;30:359-94. [Crossref] [PubMed]
- Rudkin AK, Lee AW, Chen CS. Vascular risk factors for central retinal artery occlusion. Eye (Lond) 2010;24:678-81. [Crossref] [PubMed]
- Varma DD, Cugati S, Lee AW, et al. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond) 2013;27:688-97. [Crossref] [PubMed]
- Fineman MS, Savino PJ, Federman JL, et al. Branch retinal artery occlusion as the initial sign of giant cell arteritis. Am J Ophthalmol 1996;122:428-30. [Crossref] [PubMed]
- Bossert M, Prati C, Balblanc JC, et al. Aortic involvement in giant cell arteritis: current data. Joint Bone Spine 2011;78:246-51. [Crossref] [PubMed]
- Dasgupta B, Borg FA, Hassan N, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford) 2010;49:1594-7. [Crossref] [PubMed]
- Brown GC, Magargal LE, Shields JA, et al. Retinal arterial obstruction in children and young adults. Ophthalmology 1981;88:18-25. [Crossref] [PubMed]
- Bruno A, Jones WL, Austin JK, et al. Vascular outcome in men with asymptomatic retinal cholesterol emboli. A cohort study. Ann Intern Med 1995;122:249-53. [Crossref] [PubMed]
- Chang SH, Miller NR. The incidence of vision loss due to perioperative ischemic optic neuropathy associated with spine surgery: the Johns Hopkins Hospital Experience. Spine (Phila Pa 1976) 2005;30:1299-302. [Crossref] [PubMed]
- Sys J, Michielsen J, Mertens E, et al. Central retinal artery occlusion after spinal surgery. Eur Spine J 1996;5:74-5. [Crossref] [PubMed]
- Park SW, Woo SJ, Park KH, et al. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol 2012;154:653-62.e1.
- Callizo J, Feltgen N, Pantenburg S, et al. Cardiovascular Risk Factors in Central Retinal Artery Occlusion: Results of a Prospective and Standardized Medical Examination. Ophthalmology 2015;122:1881-8. [Crossref] [PubMed]
- Douglas DJ, Schuler JJ, Buchbinder D, et al. The association of central retinal artery occlusion and extracranial carotid artery disease. Ann Surg 1988;208:85-90. [Crossref] [PubMed]
- Klein R, Klein BE, Moss SE, et al. Retinal emboli and cardiovascular disease: the Beaver Dam Eye Study. Arch Ophthalmol 2003;121:1446-51. [Crossref] [PubMed]
- Tanaka K, Uehara T, Kimura K, et al. Comparison of Clinical Characteristics among Subtypes of Visual Symptoms in Patients with Transient Ischemic Attack: Analysis of the PROspective Multicenter registry to Identify Subsequent cardiovascular Events after TIA (PROMISE-TIA) Registry. J Stroke Cerebrovasc Dis 2018;27:1711-6. [Crossref] [PubMed]
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064-89. [Crossref] [PubMed]
- Petzold A, Islam N, Hu HH, et al. Embolic and nonembolic transient monocular visual field loss: a clinicopathologic review. Surv Ophthalmol 2013;58:42-62. [Crossref] [PubMed]
- . Current management of amaurosis fugax. The Amaurosis Fugax Study Group. Stroke 1990;21:201-8. [Crossref] [PubMed]
- Pula JH, Kwan K, Yuen CA, et al. Update on the evaluation of transient vision loss. Clin Ophthalmol 2016;10:297-303. [Crossref] [PubMed]
- Hayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology 2009;116:1188-94.e1-4.
- Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina 2007;27:276-89. [Crossref] [PubMed]
- Beatty S, Au Eong KG. Acute occlusion of the retinal arteries: current concepts and recent advances in diagnosis and management. J Accid Emerg Med 2000;17:324-9. [Crossref] [PubMed]
- Shinoda K, Yamada K, Matsumoto CS, et al. Changes in retinal thickness are correlated with alterations of electroretinogram in eyes with central retinal artery occlusion. Graefes Arch Clin Exp Ophthalmol 2008;246:949-54. [Crossref] [PubMed]
- Hayreh SS, Jonas JB. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol 2000;129:786-95. [Crossref] [PubMed]
- Rudkin AK, Lee AW, Chen CS. Ocular neovascularization following central retinal artery occlusion: prevalence and timing of onset. Eur J Ophthalmol 2010;20:1042-6. [Crossref] [PubMed]
- Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion. II. Occurrence in central and branch retinal artery occlusion. Arch Ophthalmol 1982;100:1585-96. [Crossref] [PubMed]
- Mason JO 3rd, Patel SA, Feist RM, et al. Ocular neovascularization in eyes with a central retinal artery occlusion or a branch retinal artery occlusion. Clin Ophthalmol 2015;9:995-1000. [Crossref] [PubMed]
- Vu HT, Keeffe JE, McCarty CA, et al. Impact of unilateral and bilateral vision loss on quality of life. Br J Ophthalmol 2005;89:360-3. [Crossref] [PubMed]
- Hankey GJ, Slattery JM, Warlow CP. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ 1991;302:499-504. [Crossref] [PubMed]
- Helenius J, Arsava EM, Goldstein JN, et al. Concurrent acute brain infarcts in patients with monocular visual loss. Ann Neurol 2012;72:286-93. [Crossref] [PubMed]
- Park SJ, Choi NK, Yang BR, et al. Risk and Risk Periods for Stroke and Acute Myocardial Infarction in Patients with Central Retinal Artery Occlusion. Ophthalmology 2015;122:2336-43.e2.
- Rim TH, Han J, Choi YS, et al. Retinal Artery Occlusion and the Risk of Stroke Development: Twelve-Year Nationwide Cohort Study. Stroke 2016;47:376-82. [Crossref] [PubMed]
- Plant GT, Landau K. Thrombolysis for central retinal artery occlusion. J Neurol Neurosurg Psychiatry 2005;76:160-1. [Crossref] [PubMed]
- Klein R, Klein BE, Jensen SC, et al. Retinal emboli and stroke: the Beaver Dam Eye Study. Arch Ophthalmol 1999;117:1063-8. [Crossref] [PubMed]
- Wang JJ, Cugati S, Knudtson MD, et al. Retinal arteriolar emboli and long-term mortality: pooled data analysis from two older populations. Stroke 2006;37:1833-6. [Crossref] [PubMed]
- Chang YS, Jan RL, Weng SF, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol 2012;154:645-52.e1. [Crossref] [PubMed]
- Biousse V. Acute retinal arterial ischemia: an emergency often ignored. Am J Ophthalmol 2014;157:1119-21. [Crossref] [PubMed]
- Olsen TW, Pulido JS, Folk JC, et al. Retinal and Ophthalmic Artery Occlusions Preferred Practice Pattern(R). Ophthalmology 2017;124:120-43. [Crossref] [PubMed]
- Lee J, Kim SW, Lee SC, et al. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol 2014;157:1231-8. [Crossref] [PubMed]
- Biousse V, Trobe JD. Transient monocular visual loss. Am J Ophthalmol 2005;140:717-21. [Crossref] [PubMed]
- Lauda F, Neugebauer H, Reiber L, et al. Acute Silent Brain Infarction in Monocular Visual Loss of Ischemic Origin. Cerebrovasc Dis 2015;40:151-6. [Crossref] [PubMed]
- Golsari A, Bittersohl D, Cheng B, et al. Silent Brain Infarctions and Leukoaraiosis in Patients With Retinal Ischemia: A Prospective Single-Center Observational Study. Stroke 2017;48:1392-6. [Crossref] [PubMed]
- Greven CM, Slusher MM, Weaver RG. Retinal arterial occlusions in young adults. Am J Ophthalmol 1995;120:776-83. [Crossref] [PubMed]
- Abel AS, Suresh S, Hussein HM, et al. Practice Patterns After Acute Embolic Retinal Artery Occlusion. Asia Pac J Ophthalmol (Phila) 2017;6:37-9. [Crossref] [PubMed]
- Atkins EJ, Bruce BB, Newman NJ, et al. Translation of clinical studies to clinical practice: survey on the treatment of central retinal artery occlusion. Am J Ophthalmol 2009;148:172-3. [Crossref] [PubMed]
- Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: Follow the Guidelines. Ophthalmology 2018. [Epub ahead of print].
- Fraser SG, Adams W. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev 2009;CD001989 [PubMed]
- Almeida DR, Mammo Z, Chin EK, et al. Surgical Embolectomy for Fovea-Threatening Acute Retinal Artery Occlusion. Retin Cases Brief Rep 2016;10:331-3. [PubMed]
- Gilbert AL, Choi C, Lessell S. Acute Management of Central Retinal Artery Occlusion. Int Ophthalmol Clin 2015;55:157-66. [Crossref] [PubMed]
- Pielen A, Pantenburg S, Schmoor C, et al. Predictors of prognosis and treatment outcome in central retinal artery occlusion: local intra-arterial fibrinolysis vs. conservative treatment. Neuroradiology 2015;57:1055-62. [Crossref] [PubMed]
- Augsburger JJ, Magargal LE. Visual prognosis following treatment of acute central retinal artery obstruction. Br J Ophthalmol 1980;64:913-7. [Crossref] [PubMed]
- Reynard M, Hanscom TA. Neodymium:yttrium-aluminum-garnet laser arteriotomy with embolectomy for central retinal artery occlusion. Am J Ophthalmol 2004;137:196-8. [Crossref] [PubMed]
- Opremcak E, Rehmar AJ, Ridenour CD, et al. Restoration of retinal blood flow via translumenal Nd:YAG embolysis/embolectomy (TYL/E) for central and branch retinal artery occlusion. Retina 2008;28:226-35. [Crossref] [PubMed]
- Rudkin AK, Lee AW, Aldrich E, et al. Clinical characteristics and outcome of current standard management of central retinal artery occlusion. Clin Experiment Ophthalmol 2010;38:496-501. [Crossref] [PubMed]
- Rassam SM, Patel V, Kohner EM. The effect of acetazolamide on the retinal circulation. Eye (Lond) 1993;7:697-702. [Crossref] [PubMed]
- Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology 1995;102:2029-34; discussion 2034-5. [Crossref] [PubMed]
- Landa E, Rehany U, Rumelt S. Visual functions following recovery from non-arteritic central retinal artery occlusion. Ophthalmic Surg Lasers Imaging 2004;35:103-8. [PubMed]
- Incandela L, Cesarone MR, Belcaro G, et al. Treatment of vascular retinal disease with pentoxifylline: a controlled, randomized trial. Angiology 2002;53:S31-4. [PubMed]
- Iwafune Y, Yoshimoto H. Clinical use of pentoxifylline in haemorrhagic disorders of the retina. Pharmatherapeutica 1980;2:429-38. [PubMed]
- Arend O, Harris A, Martin BJ, et al. Retinal blood velocities during carbogen breathing using scanning laser ophthalmoscopy. Acta Ophthalmol (Copenh) 1994;72:332-6. [Crossref] [PubMed]
- Harino S, Grunwald JE, Petrig BJ, et al. Rebreathing into a bag increases human retinal macular blood velocity. Br J Ophthalmol 1995;79:380-3. [Crossref] [PubMed]
- Deutsch TA, Read JS, Ernest JT, et al. Effects of oxygen and carbon dioxide on the retinal vasculature in humans. Arch Ophthalmol 1983;101:1278-80. [Crossref] [PubMed]
- Beiran I, Goldenberg I, Adir Y, et al. Early hyperbaric oxygen therapy for retinal artery occlusion. Eur J Ophthalmol 2001;11:345-50. [Crossref] [PubMed]
- Anderson B Jr, Saltzman HA, Heyman A. The Effects of Hyperbaric Oxygenation on Retinal Arterial Occlusion. Arch Ophthalmol 1965;73:315-9. [Crossref] [PubMed]
- Cope A, Eggert JV, O'Brien E. Retinal artery occlusion: visual outcome after treatment with hyperbaric oxygen. Diving Hyperb Med 2011;41:135-8. [PubMed]
- Menzel-Severing J, Siekmann U, Weinberger A, et al. Early hyperbaric oxygen treatment for nonarteritic central retinal artery obstruction. Am J Ophthalmol 2012;153:454-9.e2. [Crossref] [PubMed]
- Feltgen N, Neubauer A, Jurklies B, et al. Multicenter study of the European Assessment Group for Lysis in the Eye (EAGLE) for the treatment of central retinal artery occlusion: design issues and implications. EAGLE Study report no. 1: EAGLE Study report no. 1. Graefes Arch Clin Exp Ophthalmol 2006;244:950-6. [Crossref] [PubMed]
- Page PS, Khattar NK, White AC, et al. Intra-Arterial Thrombolysis for Acute Central Retinal Artery Occlusion: A Systematic Review and Meta-Analysis. Front Neurol 2018;9:76. [Crossref] [PubMed]
- Ahn SJ, Kim JM, Hong JH, et al. Efficacy and safety of intra-arterial thrombolysis in central retinal artery occlusion. Invest Ophthalmol Vis Sci 2013;54:7746-55. [Crossref] [PubMed]
- Pettersen JA, Hill MD, Demchuk AM, et al. Intra-arterial thrombolysis for retinal artery occlusion: the Calgary experience. Can J Neurol Sci 2005;32:507-11. [PubMed]
- Agarwal N, Gala NB, Karimi RJ, et al. Current endovascular treatment options for central retinal arterial occlusion: a review. Neurosurg Focus 2014;36:E7 [Crossref] [PubMed]
- Noble J, Weizblit N, Baerlocher MO, et al. Intra-arterial thrombolysis for central retinal artery occlusion: a systematic review. Br J Ophthalmol 2008;92:588-93. [Crossref] [PubMed]
- Aldrich EM, Lee AW, Chen CS, et al. Local intraarterial fibrinolysis administered in aliquots for the treatment of central retinal artery occlusion: the Johns Hopkins Hospital experience. Stroke 2008;39:1746-50. [Crossref] [PubMed]
- Hattenbach LO, Kuhli-Hattenbach C, Scharrer I, et al. Intravenous thrombolysis with low-dose recombinant tissue plasminogen activator in central retinal artery occlusion. Am J Ophthalmol 2008;146:700-6. [Crossref] [PubMed]
- Nowak RJ, Amin H, Robeson K, et al. Acute central retinal artery occlusion treated with intravenous recombinant tissue plasminogen activator. J Stroke Cerebrovasc Dis 2012;21:913.e5-8. [Crossref] [PubMed]
- Arnold M, Koerner U, Remonda L, et al. Comparison of intra-arterial thrombolysis with conventional treatment in patients with acute central retinal artery occlusion. J Neurol Neurosurg Psychiatry 2005;76:196-9. [Crossref] [PubMed]
- Hwang G, Woo SJ, Jung C, et al. Intra-arterial thrombolysis for central retinal artery occlusion: two cases report. J Korean Med Sci 2010;25:974-9. [Crossref] [PubMed]
- Biousse V, Calvetti O, Bruce BB, et al. Thrombolysis for central retinal artery occlusion. J Neuroophthalmol 2007;27:215-30. [Crossref] [PubMed]
- Mercier J, Kastler A, Jean B, et al. Interest of local intra-arterial fibrinolysis in acute central retinal artery occlusion: Clinical experience in 16 patients. J Neuroradiol 2015;42:229-35. [Crossref] [PubMed]
- Biousse V. Thrombolysis for acute central retinal artery occlusion: is it time? Am J Ophthalmol 2008;146:631-4. [Crossref] [PubMed]
- Egan RA, Van Stavern R. Should Patients With Acute Central Retinal Artery Occlusion Be Treated With Intra-arterial t-PA? J Neuroophthalmol 2015;35:205-9. [Crossref] [PubMed]
- Chen CS, Lee AW, Campbell B, et al. Efficacy of intravenous tissue-type plasminogen activator in central retinal artery occlusion: report from a randomized, controlled trial. Stroke 2011;42:2229-34. [Crossref] [PubMed]
- Dumitrascu OM, Shen JF, Kurli M, et al. Is Intravenous Thrombolysis Safe and Effective in Central Retinal Artery Occlusion? A Critically Appraised Topic. Neurologist 2017;22:153-6. [Crossref] [PubMed]
- Page PS, Cambon AC, James RF. Visual Improvement after Intra-Arterial Thrombolysis for Central Retinal Artery Occlusion Does Not Correlate with Time to Treatment. Interv Neurol 2016;5:131-9. [Crossref] [PubMed]
- Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology 2010;117:1367-75.e1.
- Preterre C, Godeneche G, Vandamme X, et al. Management of acute central retinal artery occlusion: Intravenous thrombolysis is feasible and safe. Int J Stroke 2017;1747493016687578 [PubMed]
- Streifler JY, Eliasziw M, Benavente OR, et al. The risk of stroke in patients with first-ever retinal vs hemispheric transient ischemic attacks and high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Arch Neurol 1995;52:246-9. [Crossref] [PubMed]
- Kvickstrom P, Lindblom B, Bergstrom G, et al. Amaurosis fugax - delay between symptoms and surgery by specialty. Clin Ophthalmol 2016;10:2291-6. [Crossref] [PubMed]
- Naylor AR, Robinson TG, Eveson D, et al. An audit of management practices in patients with suspected temporary monocular blindness. Br J Ophthalmol 2014;98:730-3. [Crossref] [PubMed]

张荣沛
中山大学中山眼科中心.眼科学博士(在读),中山大学中山眼科中心2021级博士研究生。本科毕业于山东第一医科大学,硕士毕业于大连医科大学,以第一作者发表SCI论文一篇。硕士毕业课题研究方向为白内障,目前主要从事眼整形方向的相关研究。(更新时间:2021/8/12)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Dattilo M, Newman NJ, Biousse V. Acute retinal arterial ischemia. Ann Eye Sci 2018;3:28.


