
Structural analysis of processed corneas
Introduction
One of the key challenges for a tissue engineer to overcome is to translate the designed tissue/organ into a transplantable product. Although this goal is clear and practical, it is rarely achieved because the artificial tissue would either be damaged or contaminated during the end-point quality check before it is used directly in surgery. This critical issue impacts several sectors, including manufacture, quality control, regulation, and treatment application. Without a satisfactory solution, patients cannot benefit from the fruit of tissue engineering.
This challenge is particularly difficult to surmount when the scaffold in question is an artificial, decellularized animal cornea. Artificial corneas, if held in reserve in all hospitals, can be used for emergency treatments of various causes (1). Typically, the stroma region of an animal cornea, which constitutes 90% of the thickness of a cornea body (2), is utilized to design a decellularized cornea. During the manufacture, incomplete decellularization processes are frequently observed. Residual debris or animal cells can cause immune responses after the surgery. On the other hand, the transplanted corneas, however, must also display satisfactory transparency at all time. The manufacturing process may result in structural or material damage of the lamella collagen, easily causing the artificial cornea to lose transparency (3-5). For this reason, the decellularization process must be optimized to deliver a clean corneal scaffold with intact microstructures. The product then needs to go through sterilization and be vacuum-packed for the later surgery. Before the surgery takes place, the scaffold should be 85% rehydrated, with a higher amount of water toward the posterior end (6). The whole process, including material acquisition, manufacture, packaging, and rehydration, has to be error-free to maintain a fully functional corneal scaffold. Examination results before packaging cannot be carried over to guarantee a well maintained final product.
Some desirable features, when combined, can address this issue and solve the problem for product translation. First, it is crucial to include a non-invasive, non-destructive method for the end-point investigation. This method is important because there is no remedy if the corneal product is destroyed or contaminated during the characterization. With its unique triple-helix structure, collagen fibers in a decellularized scaffold are capable of generating second harmonic generation (SHG) signals from an incidental laser (7). This elastic scattering property may provide a unique opportunity for developing a non-invasive strategy. Second, the tissue scaffold should be evaluated in a global and thorough manner. Despite the fact that such a product may be ready for transplantation, the investigation at this point should still provide us enough information regarding the whole structure and content of the scaffold. Partial and random analysis of the product is insufficient and will put the patient’s health at risk. Third, the analysis should be scientifically sound, with quantifiable data for statistics. This may be achieved with several existing image processing tools, particularly the Isosurface (ISO) module (8-13). The tissue product should be free from any subjective determination. Quantitative analysis can be transformed into a reliable quality control methodology. Lastly, the method should help determine whether a corneal scaffold can be fully hydrated and become transparent right before the transplantation. This would provide the highest success rate for the surgery.
In order to meet the above requirements, we investigated into the idea that SHG imaging of the fibrillar collagen, when coupled with some robust image analytic tools, can prove to be a powerful solution to the issues found in the translational phase of tissue engineering. Multiphoton microscopy (MPM), which utilizes an infrared laser, can penetrate deep into the tissue without impairing the scaffold (14). The mode-locked laser also provides an extra spatial control to exclude out-of-plane noises. For this reason, it would be an ideal light source for visualizing the stroma region of the corneas. It is hypothesized here that this non-destructive three-dimensional (3D) structural analysis can be used to select for a corneal scaffold that can be rehydrated successfully before the transplantation.
Methods
A rehydration process was used to demonstrate how analytical this method can be for providing a reliable, non-destructive examination for a transplantable cornea. Three processed corneas were provided by our collaborator China Regenerative Medicine International (CRMI) to undergo rehydration in phosphate-buffered saline (PBS). The 3D images of decellularized corneal samples were acquired by a multiphoton microscope with an 800 nm laser. Signals were collected with either a 20× or 60× objective from three channels with optical filters at 433, 520, and 667 nm. The z-step was 0.45 µm, and scan speed was 166 lines-per-second (LPS). Images were taken and quantified when the scaffolds were dry, and then were retaken on the 4th and the 72nd hours during rehydration to assess any change in the collagen organization.
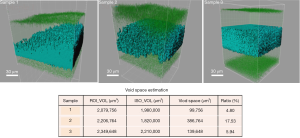
The images were then blindly deconvolved using AutoQuant X3 and analyzed with Imaris 7 (15). Collagen microstructures were reconstructed using the ISO modeling, which utilized a marching cube algorithm to clearly distinguish the scaffold from the unoccupied space (16). A signal look-up table was used for each 3D image to filter out the remaining noise and to set the threshold merely for the collagen signals. A cubical region of interest (ROI) was set up inside the cornea, and within the ROI volume, ISOs were constructed to encapsulate the collagen fibers. The unoccupied space, also known as the void space (V), could be calculated by VROI–VISO. This value indicates how loose the scaffold was while being imaged, which would give us a good starting point to predict what would happen during rehydration.
Results
By analyzing the 3D images of the dried scaffolds, we found out that there exist subtle structural differences amongst the three samples that were submitted for investigation. Figure 1 shows the transparent ROI and the ISOs that were modelled inside; the void space calculation was tabulated to provide further analysis. All three samples showed intact exterior surfaces. But when we look into the interior, Sample 1 apparently displayed unequal collagen distribution. Since the 3D images were constructed by stacking various z-planes together, the blue collagen signals should appear simultaneously if the local collagen concentrations are equal. However, it is shown by the ISO rendering that the collagen was much denser at one corner of the scaffold. The overall void space was estimated to occupy only 4.80% of the interior volume, which was the lowest amongst the three samples. Sample 2, although having more pores and concaves in the oval surface, actually had evenly distributed collagen inside the scaffold. Its void space was estimated to be 17.53%, the highest amongst the three samples. This means that there was almost 1/5 of the interior volume to be filled up during the rehydration process. Sample 3 shows highly dense and balanced collagen throughout the scaffold. Although these fibers were equally distributed, the void space analysis indicated that there might be limited space for water entry and collagen re-distribution, which would likely be reflected during rehydration.
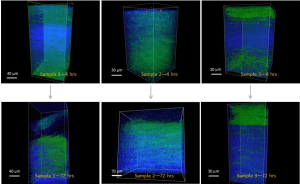
When the scaffolds were rehydrated, they started to swell when water entered the void spaces inside the scaffolds. This caused a change of the collagen organization of the scaffolds. Figure 2 demonstrates this change by featuring the images taken at hour 4 and hour 72. The three samples show different fates during rehydration. Previously our correlation indicated that 48 hours should provide enough time to completely rehydrate these dried cornea scaffolds (data not shown). Here, however, Sample 1 still had many collagen regions remain dense. The green ISOs were also unequally distributed, more concentrated at one corner. This reflects upon our dried image data in Figure 1 and shows that some of the collagen clusters would remain highly dense even if the scaffold is submerged in saline for days. This would result in an incomplete rehydration, rendering this scaffold not useful for surgery. On the other hand, Sample 2 was fully permeated after 72 hours, as shown by the ubiquitous green presence throughout the scaffold. Together with the 4th hour data, it shows that water permeated fast in Sample 2. This finding echoed our calculation from the ROI, indicating that the void space analysis may be predictive for how much a scaffold can be rehydrated. Sample 3 initially showed lots of void spaces to be occupied by water in early hours. However, only the bottom portion of the scaffold was taken by the collagen fibers from the center. The top portion of the scaffold remained unoccupied by the collagen after 72 hours. This indicates that the earlier void space analysis successfully predicted that the central collagen was too compact to loosen up during rehydration. Therefore, although collagen fibers were equally distributed in Sample 3, this type of scaffold was still not ideal to go through the rehydration for later transplantation.
Discussion
From our investigation, it was found that SHG imaging provides a solution to meet the need for an end-point evaluation of the tissue-engineered corneal scaffolds. To make sure that such a scaffold is manufactured as designed for transplantation, it is important to quantitatively evaluate the scaffold globally at the end of production (17). Because decellularized corneal stroma almost fully consists of collagen fibers, the scaffold can naturally respond to the infrared laser to initiate SHG signals. These signals do not degrade the scaffold in any manner and are strong enough to provide a good contrast to the background noise, as shown by our precise void space calculations. The capability of MPM imaging proves to be a crucial component for our hypothesis since quantifiable image analysis can only be based on the high-quality signal acquisition at the beginning of the imaging procedure. If the mosaic scanning mode can become more high-throughput, a global evaluation of the 3D structure of the corneal scaffold will be available, and MPM will become an essential tool for tissue engineering scaffold design.
The use of ISO modeling is a crucial step to turn image pixels into volumetric and quantifiable entities, which empowers and transforms the role of microscopic imaging in tissue engineering. This method enables statistical relevant decisions to be made, which is directly related to the quality control process. Furthermore, as we demonstrated, the calculated void spaces can illustrate how much water may access the interior of the scaffold. This method answers both of the questions whether a corneal scaffold is hydrated enough to be progressed into transplantation and whether such a scaffold is hydrated at the right interior location in a straightforward manner. With such a quantitative imaging method, other parameters such as the thickness of the scaffold, swelling of the scaffold, and interior collagen distribution can also be calculated for a more detailed analysis. Understanding how these parameters behave is necessary for translating a laboratory-made corneal scaffold into a reliable medical product.
Although this method that combines MPM and ISO has been proven to be highly useful, there are rooms for future improvements. First, the imaging process is time-consuming and requires the user to have precise control over the optical components, sample positioning, and signal-to-noise ratio. All these add uncertainties while efforts are made to reproduce a certain result. Hence, it is important to design an imaging platform that reduces human involvements. Second, the usage of ISO remains a point of debate, mainly due to the issue of signal thresholding. A seasoned user would have to address the selection of signals and more likely rely on automated thresholding by the image software. Doing so prevents any bias in the volumetric analysis, renders an estimated void space that will be more reliable for precise modeling. Lastly, this presented method only works for materials that are capable of emitting SHG signals. If other complex materials or tissue were to be visualized, it is necessary to utilize another optical process for the generation of a reliable, non-destructive signal. This means that the image analyst has to possess a wide understanding of optical processes, which also adds a tremendous complexity to the design of other tissues or organs.
Conclusions
In this letter, we present a non-invasive, quantitative, analytical, 3D, and universally applicable method for the analysis of the microstructure of a cornea scaffold. Our data indicated that Sample 2, which showed equally distributed collagen fibers and a higher void space, could be fully rehydrated before surgery. This experiment demonstrates that by combining MPM with image analytic tools such as ISO, we can render the 3D corneal images quantifiable and acquire useful data on how the processed cornea may respond to follow-up procedures, such as rehydration, cell seeding with epithelial cells or keratocytes, and mechanical processes, etc. It is further possible to be used as a method for the analysis of transparency, a crucial functional attribute that is highly associated with the collagen microstructure. The non-invasive nature of this method presents a great industrial potential to be used as a quality control procedure. Overall, our data demonstrated that, if made systematically, this analytic method can highly impact current tissue engineering of artificial corneal scaffolds.
Acknowledgments
The authors would like to thank China Regenerative Medicine International (CRMI) for providing the corneal scaffolds to be analyzed in this paper.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Weiyun Shi and Jin Yuan) for the series “Bioengineering Cornea” published in Annals of Eye Science. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2018.01.02). The series “Bioengineering Cornea” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- King JH Jr. The use of preserved ocular tissues for transplantation. Trans Am Ophthalmol Soc 1958;56:203-11; discussion 211-6. [PubMed]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res 2010;91:326-35. [Crossref] [PubMed]
- Fitch JM, Gross J, Mayne R, et al. Organization of collagen types I and V in the embryoin chicken cornea: monoclonal antbody studies. Proc Natl Acad Sci U S A 1984;81:2791-5. [Crossref] [PubMed]
- Knupp C, Pinali C, Lewis PN, et al. The architecture of the cornea and structural basis of its transparency. Adv Protein Chem Struct Biol 2009;78:25-49. [Crossref] [PubMed]
- Jalbert I, Stapleton F. The corneal stroma during contact lens wear. Contact Lens Anterior Eye 2005;28:3-12. [Crossref] [PubMed]
- Pircher M, Götzinger E, Leitgeb R, et al. Measurement and imaging of water concentration in human cornea with differential absorption optical coherence tomography. Opt Express 2003;11:2190-7. [Crossref] [PubMed]
- Cox G, Kable E, Jones A, et al. 3-Dimensional imaging of collagen using second harmonic generation. J Struct Biol 2003;141:53-62. [Crossref] [PubMed]
- Yerly J, Hu Y, Jones SM, et al. A two-step procedure for automatic and accurate segmentation of volumetric CLSM biofilm images. J Microbiol Methods 2007;70:424-33. [Crossref] [PubMed]
- Yang X, Beyenal H, Harkin G, et al. Quantifying biofilm structure using image analysis. J Microbiol Methods 2000;39:109-19. [Crossref] [PubMed]
- Yang X, Beyenal H, Harkin G, et al. Evaluation of biofilm image thresholding methods. Water Res 2001;35:1149-58. [Crossref] [PubMed]
- Baveye P. Comment on “Evaluation of biofilm image thresholding methods.” Water Res 2002;36:805-7. [Crossref] [PubMed]
- Beyenal H, Donovan C, Lewandowski Z, et al. Three-dimensional biofilm structure quantification. J Microbiol Methods 2004;59:395-413. [Crossref] [PubMed]
- Xavier JB, Schnell A, Wuertz S, et al. Objective threshold selection procedure (OTS) for segmentation of scanning laser confocal microscope images. J Microbiol Methods 2001;47:169-80. [Crossref] [PubMed]
- Wilder-Smith P, Osann K, Hanna N, et al. In vivo multiphoton fluorescence imaging: a novel approach to oral malignancy. Lasers Surg Med 2004;35:96-103. [Crossref] [PubMed]
- Lam EY, Goodman JW. Iterative statistical approach to blind image deconvolution. J Opt Soc Am A Opt Image Sci Vis 2000;17:1177-84. [Crossref] [PubMed]
- Lorensen WE, Cline HE. Marching cubes: A high resolution 3D surface construction algorithm. In: ACM siggraph computer graphics. New York: ACM, 1987:163-9.
- Casadessus O, Georges G, Siozade-Lamoine L, et al. Scattering properties and transparency characterization of human corneal grafts. Proc. SPIE 8091 Optical Coherence Tomography and Coherence Techniques 2011;V:80911G [Crossref]
Cite this article as: Yeh SA, Ye H, Cui Z. Structural analysis of processed corneas. Ann Eye Sci 2018;3:11.


