
Rescue with intravitreal bevacizumab in aggressive posterior retinopathy of prematurity poorly responsive to laser treatment
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative disorder of preterm babies, with vascular endothelial growth factor (VEGF) playing a pivotal role in its pathogenesis (1). Presence of raised levels of VEGF in vitreous cavity of such premature infants is the basis of use of anti-VEGF agents to arrest disease progression (2-4). Although, the results of BEAT-ROP (Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity) study have been promising in stage 3+ ROP, its use as primary modality of treatment is not universally accepted (5). More so, anti-VEGF agents have mainly been used as monotherapy or as an adjunct pre-laser in ROP (6,7). We report a case scenario where sequential combination of laser and anti-VEGF therapy gave favourable results.
Case presentation
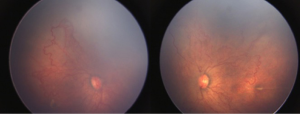
A male child delivered by lower segment caesarean section (LSCS) at 28 weeks of gestation with a birth weight of 1,200 grams, was referred for ROP screening 5 weeks after birth. He was admitted to neonatal intensive care unit (NICU) for 1 month during which he received a single blood transfusion. Anterior segment examination revealed poor pupillary dilatation, prominent engorged iris vessels associated with tunica vasculosa lentis. On fundus evaluation, there was media haze with dilated retinal veins and tortuous retinal arteries over the posterior pole along with arterial-venous shunting, circumferential vessels and large areas of avascular retina in both eyes (Figure 1). There was no definitive demarcation line/ridge or extra-retinal fibrovascular proliferation. There was plus disease which appeared out of proportion to the quiescent appearance of the avascular-vascular junction. Based on clinical findings, a diagnosis of zone 1 aggressive posterior (AP) ROP was made in both eyes.
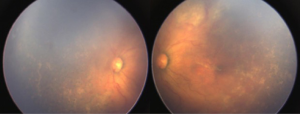
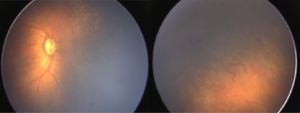
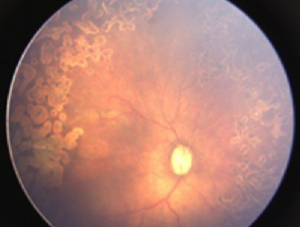
Both eyes underwent laser treatment using double frequency Nd: YAG laser to cover the entire visible avascular retina. One week following laser, media was clear and plus disease reduced in left eye (Figure 2). However, in right eye the media was still hazy and plus disease persisted (Figure 3). Persistence of plus disease with continuing media haze in right eye on 14th day post laser, prompted us to give 0.625 mg/0.025 mL intravitreal bevacizumab (IVB) under general anaesthesia (GA) in the right eye. Three days after post IVB, the media was clearer and gradual regression of plus disease was noted by day 7 (Figure 4). No adverse effects related to injection were noted during follow up. The disease regressed completely in both eyes at 1 month follow up.
Discussion
In the past years, management of ROP has seen a changing trend with surgeons shifting towards anti-VEGF agents for treating AP-ROP or zone 1 stage 3+ disease (8). Recent studies have also confirmed regression of plus disease and achievement of near normal vascularization subsequent to anti-VEGF monotherapy, which is unlikely to be achieved with laser therapy (9-11). However its use as a first line of treatment replacing laser is still not advocated in absence of randomized trials confirming long term safety (8). In spite of lower recurrence rate reported after monotherapy IVB therapy, such cases require longer follow up as recurrences have been reported as late as 5 months post injection (12).
Although, ETROP had laid guidelines for laser therapy in type 1 ROP, post laser macular ectopia and progression to stage 5 have often been noted (13). Such complications seem to be reported less in IVB treated eyes (5). Moreover in aggressive posterior retinopathy of prematurity (APROP) where disease progression is rapid, prompt treatment is mandatory to prevent otherwise irreversible vision loss. With handicaps such as inadequate pupil dilatation and hazy media, laser therapy is often limited to only visible areas. Therefore a large area of peripheral retina remains untreated in such cases due to visibility issues, and excessive ablation of the visible posterior retina, may lead to inflammation, anterior segment ischemia and cataract (14). In such a scenario, it is prudent to inject anti-VEGF agent as a second line of treatment.
In our case, the plus disease persisted even at 2 weeks post laser, hence a decision for IVB was taken to prevent unfavourable outcomes. Usually we would expect good laser response to occur from 7 to 14 days leading to clearing of the media, better pupil dilatation and regression of plus disease (6). Despite left eye showing an expected response, the right eye did not improve. The rapid effect of IVB within 48 hours is well known (5). Failure of ablative therapy has been hypothesized to be due to different mechanisms involving vasculogenesis less dependent on VEGF mediated angiogenesis or a second source of VEGF, such as vitreal macrophages (15,16). Intravitreal anti-VEGF agents are useful as rescue treatment after failure of laser, decreasing the VEGF load from such sources and blocking vasculogenesis.
Previous reports on combination treatment of anti-VEGF agents with laser have either had simultaneous treatment with both or with anti-VEGF given prior to laser (17,18). Thus far, there is only a single report of three cases where anti-VEGF has been given subsequent to failure of laser therapy (19). All these three babies were referred from outside, presenting late with variable course and required further laser ablation following anti-VEGF injection. Our case did not require any additional therapy after injection, as it was probably given within an interval where it would be most effective. Hence we propose, failure of laser therapy can be rescued with intravitreal injection of anti-VEGF within a short window period, probably within 2 weeks, before tractional detachment ensues and further laser ablation may not be required thereafter.
Conclusions
In absence of randomized control trials with long term safety profile of anti-VEGF agents in premature babies, IVB may be a useful alternative as a rescue therapy for disease control after failure of initial laser therapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2017.12.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed written consent was obtained for all the procedures involved in management.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995;1:1024-8. [Crossref] [PubMed]
- Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007;114:855-9. [Crossref] [PubMed]
- Travassos A, Teixeira S, Ferreira P, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging 2007;38:233-7. [PubMed]
- Geloneck MM, Chuang AZ, Clark WL, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol 2014;132:1327-33. [Crossref] [PubMed]
- Mintz-Hittner HA, Kennedy KA, Chuang AZ, et al. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603-15. [Crossref] [PubMed]
- Hwang CK, Hubbard GB, Hutchinson AK, et al. Outcomes after Intravitreal Bevacizumab versus Laser Photocoagulation for Retinopathy of Prematurity: A 5-Year Retrospective Analysis. Ophthalmology 2015;122:1008-15. [Crossref] [PubMed]
- Chung EJ, Kim JH, Ahn HS, et al. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2007;245:1727-30. [Crossref] [PubMed]
- Pertl L, Steinwender G, Mayer C, et al. A Systematic Review and Meta-Analysis on the Safety of Vascular Endothelial Growth Factor (VEGF) Inhibitors for the Treatment of Retinopathy of Prematurity. PLoS One 2015;10:e0129383 [Crossref] [PubMed]
- Castellanos MA, Schwartz S, García-Aguirre G, et al. Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br J Ophthalmol 2013;97:816-9. [Crossref] [PubMed]
- Chen SN, Lian I, Hwang YC, et al. Intravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between Ranibizumab and Bevacizumab. Retina 2015;35:667-74. [Crossref] [PubMed]
- Salman AG, Said AM. Structural, Visual and Refractive Outcomes of Intravitreal Aflibercept Injection in High-Risk Prethreshold Type 1 Retinopathy of Prematurity. Ophthalmic Res 2015;53:15-20. [Crossref] [PubMed]
- Patel RD, Blair MP, Shapiro MJ, et al. Significant treatment failure with intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol 2012;130:801-2. [Crossref] [PubMed]
- Good WVEarly Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233-48; discussion 248-50. [PubMed]
- Stuart A. Current ROP Therapies: How Laser and Anti-VEGF Compare. EyeNet 2014:38-44.
- Flynn JT, Chan-Ling T. Retinopathy of prematurity: Two distinct mechanisms that underlie zone 1 and zone 2 disease. Am J Ophthalmol 2006;142:46-59. [Crossref] [PubMed]
- Naug HL, Browning J, Gole GA, et al. Vitreal macrophages express VEGF165 in oxygen-induced retinopathy. Clin Exp Ophthalmol 2000;28:48-52. [Crossref] [PubMed]
- Law JC, Recchia FM, Morrison DG, et al. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. J AAPOS 2010;14:6-10. [Crossref] [PubMed]
- Chung EJ, Kim JH, Ahn HS, et al. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2007;245:1727-30. [Crossref] [PubMed]
- Lalwani GA, Berrocal AM, Murray TG, et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina 2008;28:S13-8. [Crossref] [PubMed]
Cite this article as: Kumar V, Kinkhabwala R, Chandra P, Takkar B, Azad SV, Azad R. Rescue with intravitreal bevacizumab in aggressive posterior retinopathy of prematurity poorly responsive to laser treatment. Ann Eye Sci 2018;3:9.


