
Limbal epithelial stem cells in corneal surface reconstruction
Limbal epithelial stem cell (LESC) and the niche
Cornea, the transparent dome-shaped structure residing at the anterior frontline of the eye, has several fundamental functions in vision. As a front barrier, cornea protects all the other eye structures from external damage combined with other region of fibrous tunic. Cornea also serves as the anatomical feature for light transmission and fraction, which are crucial steps for image formation. The light transmission and fraction relied deeply on the transparency and curvature of the cornea (1).
LESCs are a group of somatic stem cells that constantly asymmetrically divide, differentiate and centripetally migrate toward cornea center to replenish the worn-out corneal epithelial cells (CEpCs) (2). CEpCs, as consist as the outermost layer of the cornea, maintain the avascular and dehydrated feature for cornea transparency and integrity so that clear vision can be kept. As an important source of CEpCs, in vivo LESCs are always in continuous differentiating stages which makes it very difficult to have definitive stem cell markers. Some promising maker candidates emerged by recent studies, including ABCB5 (3), ABCG2 (4) and several cytoskeletal proteins like K14, K15, and K19 (5,6). Although they provide helps to distinguish between stem cell, but progenitor and transit-amplifying cell is still limited. A nuclear protein p63 was also identified as a putative LESC marker by Pellegrini et al. in 2001 (7). Connexin 43, a gap junction protein, expresses in differentiated CEpCs but is absent in LESCs or limbal basal epithelium (8,9). This expression property of connexin 43 makes it a credible negative marker for LESCs, which can help us distinguish limbal and CEpCs with diverse differentiation stages.
LESCs situated at limbus, specifically a transition zone of cornea and conjunctiva called palisades of Vogt (PV). The PV area is composed of radial fibrovascular ridges and also accepted as the limbal stem cell niche (LSCN). Healthy LSCN could provide ideal environment for homeostasis of LESCs, including supporting cells, extracellular matrix (ECM) and growth factors etc. Recently other major signaling pathways like Notch, TGF-β/BMP and Wnt/β-catenin have been reported to be involved in the regulation of LSCN (10-13). Loss of Wnt inhibitor Dkk2 could not only upregulate Wnt/β-catenin signaling in limbal stoma but also decrease PAX6 expression in corneal epithelium (14), which is the key transcription factor in cornea development. Results provided by this research proved us the involvement of Wnt and PAX6 in the regulation of LESCs in niche (14), and another study using PAX6+/− mice model also indicated the key function of PAX6 in LSCN dysfunction (15). Keratinocyte growth factor (KGF) is another important molecule that can help LSCN maintaining function, overexpression of KGF will lead to abnormal proliferation and differentiation of LESCs (16). Certain cytokine related pathway (SDF-1/CXCR4) have been proved to be associated with limbal niche maintenance (17).
Limbal basement membrane expresses a series of laminin and collagen family proteins including laminin-1, laminin-5 and type IV collagen (α1, α2 and α5 chains), all these ECM components might help stem cells to distribute normally in the niche (18-20). Regulation by supramolecular micro-environmental of these ECM is another type of mechanism that can determine the fate of LESCs besides the aforementioned intracellular signaling pathway. Aldrovani et al. (21) carefully reviewed recent progresses of LESCs regulation by biophysical and mechanical cues in LSCN. ECM may form as shape of liquid crystalline superstructure to have active physiological functions, and the superstructure could be ECM macromolecular complexes including fibrils, fibers and lamellae etc. Multiple kinds of cells and the closeness of vasculature in the basal layer of limbus are also key factors for LESCs regulation in LSCN (22). The mystery of crosstalk between LESCs and melanocytes, Langerhans cells, suppressor T-lymphocytes or other kind of cells in the niche is yet to be unveiled, and the future discovery of these dialogues will surely facilitate us understanding the refined regulation of LESCs in the niche (20).
Recently, a growing number of independent studies showed that corneal regeneration could happen in central corneas without limbus involvement. These phenomena indicate that central corneas could be the “second niche” of LESCs. Several studies form animal models showed that cornea homeostasis could be maintained after artificial limbal destruction which indicate the existence of LESCs in the central part of cornea. However, there is less evidence showed long-term corneal epithelial regeneration effect in limbus deficient model and the direct observation of LESCs in the central cornea has not been reported yet (23-26). Researchers suspect that the corneal epithelial regeneration in the central cornea could be the contribution of transient-amplifying cell (TAC) migrated from limbus (27). Given the current study situation, the secondary niche theory of LECSs is indeed promising and interesting, but more direct fundamental evidence is also required for this theory to be more persuasive.
Limbal stem cell deficiency (LSCD)
LSCD is a disease defined by the irreversible impairment and dysfunction of LESCs and its essential supporting structure (niche) in the limbus. In the absence of LESCs and LSCN, the cornea loses its regeneration ability towards epithelium. Besides this, neovascularization and conjunctivalization in the cornea can also be recognized as the feature of LSCD by microscopy in pathology respect. The diagnostic modality of LSCD is currently poor and relies on the clinical observation. Impression cytology, which involves transferring, blotting and immunostaining of superficial ocular surface cells, is currently the best option for LSCD identification.
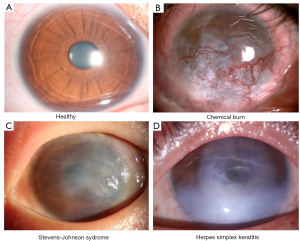
The clinical symptoms of LSCD observed from patients include chronic ocular surface discomfort, vision loss, and photophobia. The causes of LSCD varies and can be categorized to two groups: the extrinsic causes like chemical burn, radiation and infections like herpes simplex keratitis etc.; the intrinsic causes like Stevens-Johnson syndrome (SJS), aniridia, and chronic limbitis etc. (Figure 1). LSCD can also be categorized by the occasion site (unilaterally or bilaterally) or order of severity (partially or totally). The categorization of LSCD for patients decides the technique selection for the treatment. For partial LSCD that the center part of the cornea is relatively intact, conservative measures should be chosen for treatment (28). While in cases of total LSCD no matter unilateral or bilateral, surgery is indispensable.
Tissue transplant therapy for LSCD
As mentioned above, LSCD patients with different causes, severity, occasion sites, or involvement of conjunctiva should choose customized therapy for optimal therapeutic effect. To ameliorate the suffering of LSCD beside the surgery, medical management with the medicines should be considered, like topical lubricants, corticosteroids or autologous serum drops (29). For partial LSCD, corneal surface can be easily restored by conjunctival epitheliectomy with biological substrate. While in more severe or total LSCD case, tissue transplantation should be considered as the prime method. In next part of this section, tissue-based transplant therapies will be described: conjunctival limbal autograft (CLAU), limbal allograft (LAL) and keratoprosthesis (Kpro).
CLAU
In patients with unilateral total LSCD, the CLAU technique directly apply relatively larger conjunctival limbal tissue from the other healthy eye for autologous transplantation. As one of the curative techniques for LSCD, CLAU was first applied and described by Kenyon et al. back to 1989 (30). CLAU supplies appropriate amount of LESCs for corneal surface restoration, and takes no risk for immune rejection. The long-term success rate of CLAU therapeutic procedure can reach to 82% (31).
Despite the relative high success rate, the risk for the healthy eye should take into consideration prior to CLAU operation. CLAU is a preferable technique for unilateral total LSCD, but the visual improvement is greatly relied on the area of the CLAU graft. A recent research showed that the visual improvement rate of smaller grafts is 30% lower than CLAU with large grafts (32). Better surgical skills or newer techniques should be developed to achieve the best medical effect of the affected eye and avoid the risk of the donor healthy eye in the meantime.
LAL
For patients with bilateral LSD that none or less autograft is available for transplantation, LAL can be used as an alternative method for corneal surface reconstruction (33). The source of conjunctival tissue of LAL can be from living parents, siblings or cadaveric limbal tissue. The tissue amount from living relative is still as limited as CLAU for the health concern of the donor eye, but limbal allograft from cadaveric tissue could provide the whole limbal and corneal structure for transplantation which will give greater stem cell supply for reconstruction.
Comparing to CLAU therapy aforementioned, LAL requires systemic immunosuppression to prevent immune rejection, and long-term usage of medicine for immunosuppression will give rise to side effects like anemia, elevated creatinine and hyperglycemia etc. (34). Even with immunosuppression, the long-term success rate of LAL therapy is not as ideal as autologous transplantation (35,36).
For patients who are not suitable or available for any therapy mentioned above, Kpro using bioengineering artificial materials provide the last choice. Depends on the severity of the LSCD, patient can make the choice of Kpro. The bilateral LSCD patient can choose Boston Kpro type 1 as the surgical option in case that immunosuppression is not applicable (37).
Cell transplant therapy for LSCD
The treatment of LSCD is gradually mature by the recent progress in surgical technique and regenerative medicine. To restore the LESCs and LSN as the ultimate aim for LSCD treatment, regenerative medicine, which is defined by using somatic stem cells to generate biological substitutes and improve tissue functions (38), have become the most promising strategy for LSCD management in the last three decades.
Cultivated limbal epithelial transplantation (CLET)
Regenerative cell therapy using somatic stem cells has gained great attention in multiple diseases treatment in recent years, especially in ocular field. In 1997, Pellegrini et al. firstly applied CLET for the ocular disease (39), in the study autologous human corneal epithelium was co-cultured with mouse fibroblast feeder layer and then transplanted into unilateral two LSCD patients. In 2001, Kolli et al. established a method that using human amniotic membrane (hAM) as substrate to facilitate cell expansion. The method is using feeder-free culture conditions with no animal product, and eight patients were successfully treated by this new technique (40). Mariappan et al. also published a standard protocol for expansion of human limbal epithelial cells in vitro in the same time, which is also a feeder-free technique using hAM as the culture substrate (41). Now CLET is one of the most mature and wildly used stem cell therapies which have been reported. European Medicine Agency (EMA) has approved CLET as legal treatment for corneal burns in 2015 (Holoclar, Holostem Terapie Avanzate, Modena, Italy).
During the general procedure of CLET technique, a small limbal biopsy is performed to harvest healthy LESCs from the donor eye firstly, and then the harvested LESCs are cultured and expanded ex vivo. When the in vitro cells reached the amount for transplantation, carriers as amniotic membrane or other substrate will be simultaneously applied to support the transplantation CLET. Depending on diverse LESCs sources, this technique has been wildly used in unilateral, partial, and total LSCD.
The most important advantage of CLET is that it requires significantly little amount of LESCs to achieve viable therapeutic effect. CLET technique can also improve epithelialization rate and reduce the risk of inflammation comparing to other transplantation techniques. To break the limit of the CLET source, both autologous and allogeneic LESCs were verified in clinical trials (42-45). As we can speculate, autologous cells are optimum source for CLET as autologous tissues require no systemic immunosuppression. Baylis et al. reviewed and summarized case series and reports of human limbal epithelium transplantation from 1997 to 2011, the combined average success rate of all allogenic and autogenic CLET is 76% by that time (44). This review also pointed that the success rate differs in the causes of LSCD, inflammatory diseases and chemical/thermal burns have obviously higher chance to be cured than congenital causes. Cauchi et al. also thoroughly evaluated the benefits and adverse effects of LSCD surgical interventions by systematic literature review (43), 26 reports screened from 2,374 were appraised by different criteria. The meaningful conclusion of the review is that LSCD interventions are promising but more standardized and prospective data need to be collected as well. A more recent study based on the meta-analysis of published cases also proved the effectiveness of CLET, and clarified that there is no significant difference between allograft and autograft in success rate as firstly shown in Baylis et al.’s review (44,45). All three reviews are recommended to read if further understanding of the big LSCD treatment picture is interested (43-45).
Despite the great outcome, multiple advantages and wildly usages, the safety issues and technique challenges of CLET do exist during each step of procedure. Above all, plenty kinds of animal and/or human based products or tissue are involved in the current CLET protocols, which carry risks for the patient. Unsafety serum product, toxic or infectious agents and contamination during cell culture are all uncertain factors for CLET success. Some potential risks can be reduced by xeno-free culture protocols, which is promising but not common yet. Furthermore, good manufacturing practice (GMP) is also required for CLET practice and raises the threshold of the technique. Simple limbal epithelial transplantation (SLET) is a new approach that only use minimum limbal tissue and scatter it for tissue transplantation, which now believed to be a valid alternative for CLET, firstly described by Sangwan et al. in 2011 (46).
Cell transplantation therapy from other sources
Although CLET performs well in LSCD treatment, the limitation of its source is obvious. To overcome the shortage of LESCs, several recent studies had investigated alternative sources for transplantation. Cultivated oral mucosal epithelial transplantation (COMET) technique using epithelial cells lining the mouth (oral mucosa) was firstly developed as a promising alternative for CLET (47,48). In 2004, Nishida et al. firstly applied the transplantation of cultured autologous oral mucosal cells to four bilateral LSCD patients, and all four patients showed obvious visual improvement (48). However, the long-term effect of COMET showed disadvantage as neo-vascularization in the cornea was observed in all patients after transplantation, which severely affect the visual acuity. Inactivated mouse fibroblasts were used as the culture substrate in this study that also limit the future usage of COMET.
More and more stem cell studies have been investigated to exploring the new source for CLET. Although most of the new developed stem cell application has not reached to the clinical step, some studies already showed promising experimental results as in mesenchymal stem cells from the adipose (49) or bone marrow tissue (50). Hair-follicle-derived stem cells (51), umbilical-cord stem cells (52), and dental pulp stem cells (53) are all potential sources of stem cell transplantation therapy for LSCD. With promising future, the efficacy and clinical application for all the stem cells mentioned above is still to be verified and developed.
Human skin epithelial stem cells (SESCs) can also be developed as a putative LESCs source for LSCD treatment after certain genetic modification. PAX6 and Wnt7a have identified to be key molecules only for corneal epithelium differentiation but not epidermal keratinocytes (54). In SESCs, transgenic expression of PAX6 could induce corneal cell phenotype and K3/K12 (corneal cell marker) expression. Of note, PAX6-transduced SESCs from rabbit could efficiently restore the LESC niche and corneal epithelium in corneal damage model. This study implied that autologous SESCs can be used as LESCs source after relatively simple genetic reprogramming of few factors, and novel treatments may be achieved with the magnificent progress of gene editing method.
Induced pluripotent stem cells (iPSCs) technique has great potential for a serial of clinical applications with advantages of no risk for immuno-rejection and no ethically hurdle. In 2006, Hayashi et al. used iPSCs as a source for LESCs transplantation (55). In animal models, iPSCs can differentiated into self-formed ectodermal autonomous multi-zone (SEAM). CEpCs can be achieved from SEAM successfully and subsequently be transplanted to recover the visual function effectively. Given all the promising result achieved, disadvantages of therapy using iPSCs are also inevitable and unneglectable. GMP-grade product for iPSCs therapy need relatively high cost and expertise, no matter the oncogenesis risk during the therapy considering the immature understanding for iPSCs application.
Although various treatment options could help patient under different circumstances, a point need to be emphasized is that the effectiveness of the methods used to treat LSCD largely depends on the causative agent of the injury. The main therapies and promising studies along the history were summarized in the following table to get a clear overview for LSCD treatment (Table 1).
Table 1
| Name | Species | Autologous/allogenic | Origin | Year | Post-surgery observation |
|---|---|---|---|---|---|
| CLAU ( |
Human | Autologous | Limbal tissue | 1989 | Average 18 months |
| LAL ( |
Human | Allogenic | Limbal tissue | 1995 | Average 17.2 months |
| CLET ( |
Human | Autologous | Cultured LESCs | 1997 | More than 2 years |
| COMET ( |
Human | Autologous | Mucosal epithelium | 2004 | 14 months |
| SLET ( |
Human | Autologous | Limbal tissue | 2011 | 9.2±1.9 months |
| Kpro ( |
Artifact | Allogenic | Boston Kpro type 1 | 2011 | Up to 3 years |
| Dental pulp stem cells ( |
Human | Autologous | Dental pulp | 2009 | 3 months |
| Hair-follicle stem cells ( |
Murine | Autologous | Hair-follicle | 2011 | 7 weeks |
| Umbilical-cord lining stem cells ( |
Rabbit | Autologous | Umbilical-cord | 2015 | 3 months |
| PAX6-transduced SESCs ( |
Human/rabbit | Autologous | SESCs | 2014 | 90 days |
| Bone marrow stromal cells ( |
Human | Allogenic | Bone marrow | 2015 | 25–35 days |
| Adipose-derived mesenchymal stem cells ( |
Human | Allogenic | Adipose | 2015 | 3 months |
| iPS cell-derived ocular SEAM ( |
Human | Autologous | iPSC lines | 2016 | 14 days |
CLAU, conjunctival limbal autograft; LAL, limbal allograft; CLET, cultivated limbal epithelial transplantation; COMET, cultivated oral mucosal epithelial transplantation; SLET, simple limbal epithelial transplantation; Kpro, keratoprosthesis; SESCs, skin epithelial stem cells; iPS, induced pluripotent stem; iPSC, induced pluripotent stem cell; SEAM, self-formed ectodermal autonomous multi-zone; LSCD, limbal stem cell deficiency.
Conclusions
LESC research progressed greatly in recent years that benefits a lot for patients with corneal surface disease. However, developments still urgently needed in many respects. Identification of more specific LESC markers will profoundly help the tracing and enriching in stem cell population and facilitating the subsequent medical application. Those novels signaling pathways which can regulate homeostasis of LESCs and the niche are also need to be unveiled, which will guide the maintenance of cornea health.
Significant progress of tissue transplantation therapies has also been achieved in the past three decades, which benefited numerous patients from malignant diseases. In cornea field, the technique of CLAU, LAL, CLET and other newer techniques prevent millions of patients from blindness and also provide viable options for LSCD treatment in the future. Although development of the treatment technique is growing in an amazing pace, some difficult obstacles are eager to be overcome. In the future, new method to solve issues including large scale of xeno-free culture, GMP generalization for stem cell therapy and efficient immunosuppression with low side effect should be developed to get better solution for LSCD.
New stem cell researches in the field of LSCD treatment developed vigorously in recent years, and they not only provide new sources for cell transplantation but also new percepts for method exploring. More effort should put into this blooming research area to achieve more efficient therapeutic effect and also conquer the putative obstacles in the future.
Acknowledgments
Funding: This research is funded by Science and Technology Planning Project of Guangdong Province (No. 2015B020226003).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Weiyun Shi and Jin Yuan) for the series “Bioengineering Cornea” published in Annals of Eye Science. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2017.12.05). The series “Bioengineering Cornea” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fuest M, Yam GH, Peh GS, et al. Advances in corneal cell therapy. Regen Med 2016;11:601-15. [Crossref] [PubMed]
- Dua HS, Forrester JV. The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol 1990;110:646-56. [Crossref] [PubMed]
- Ksander BR, Kolovou PE, Wilson BJ, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014;511:353-7. [Crossref] [PubMed]
- de Paiva CS, Chen Z, Corrales RM, et al. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells 2005;23:63-73. [Crossref] [PubMed]
- Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr Eye Res 1994;13:805-14. [Crossref] [PubMed]
- Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci 2006;47:4780-6. [Crossref] [PubMed]
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 2001;98:3156-61. [Crossref] [PubMed]
- Dong Y, Roos M, Gruijters T, et al. Differential expression of two gap junction proteins in corneal epithelium. Eur J Cell Biol 1994;64:95-100. [PubMed]
- Chen Z, Evans WH, Pflugfelder SC, et al. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells 2006;24:1265-73. [Crossref] [PubMed]
- Mei H, Nakatsu MN, Baclagon ER, et al. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cells 2014;32:938-45. [Crossref] [PubMed]
- Han B, Chen SY, Zhu YT, et al. Integration of BMP/Wnt signaling to control clonal growth of limbal epithelial progenitor cells by niche cells. Stem Cell Res 2014;12:562-73. [Crossref] [PubMed]
- Chen SY, Han B, Zhu YT, et al. HC-HA/PTX3 Purified From Amniotic Membrane Promotes BMP Signaling in Limbal Niche Cells to Maintain Quiescence of Limbal Epithelial Progenitor/Stem Cells. Stem Cells 2015;33:3341-55. [Crossref] [PubMed]
- Li J, Chen SY, Zhao XY, et al. Rat Limbal Niche Cells Prevent Epithelial Stem/Progenitor Cells From Differentiation and Proliferation by Inhibiting Notch Signaling Pathway In Vitro. Invest Ophthalmol Vis Sci 2017;58:2968-76. [Crossref] [PubMed]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, et al. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 2006;133:2149-54. [Crossref] [PubMed]
- Ramaesh T, Ramaesh K, Martin Collinson J, et al. Developmental and cellular factors underlying corneal epithelial dysgenesis in the Pax6+/− mouse model of aniridia. Exp Eye Res 2005;81:224-35. [Crossref] [PubMed]
- Lovicu FJ, Kao WW, Overbeek PA. Ectopic gland induction by lens-specific expression of keratinocyte growth factor (FGF-7) in transgenic mice. Mech Dev 1999;88:43-53. [Crossref] [PubMed]
- Xie HT, Chen SY, Li GG, et al. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells 2011;29:1874-85. [Crossref] [PubMed]
- Ljubimov AV, Burgeson RE, Butkowski RJ, et al. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest 1995;72:461-73. [PubMed]
- Tuori A, Uusitalo H, Burgeson RE, et al. The immunohistochemical composition of the human corneal basement membrane. Cornea 1996;15:286-94. [Crossref] [PubMed]
- Li W, Hayashida Y, Chen YT, et al. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res 2007;17:26-36. [Crossref] [PubMed]
- Aldrovani M, Filezio MR, Laus JL. A supramolecular look at microenvironmental regulation of limbal epithelial stem cells and the differentiation of their progeny Arq Bras Oftalmol 2017;80:268-72. [Crossref] [PubMed]
- Ordonez P, Di Girolamo N. Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells 2012;30:100-7. [Crossref] [PubMed]
- Chang CY, Green CR, McGhee CN, et al. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci 2008;49:5279-86. [Crossref] [PubMed]
- Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci 1991;32:96-105. [PubMed]
- Majo F, Rochat A, Nicolas M, et al. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 2008;456:250-4. [Crossref] [PubMed]
- Kawakita T, Higa K, Shimmura S, et al. Fate of corneal epithelial cells separated from limbus in vivo. Invest Ophthalmol Vis Sci 2011;52:8132-7. [Crossref] [PubMed]
- Yoon JJ, Ismail S, Sherwin T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J Stem Cells 2014;6:391-403. [Crossref] [PubMed]
- Dua HS. The conjunctiva in corneal epithelial wound healing. Br J Ophthalmol 1998;82:1407-11. [Crossref] [PubMed]
- Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol 2001;85:1188-97. [Crossref] [PubMed]
- Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989;96:709-22; discussion 722-3. [Crossref] [PubMed]
- Nakamura T, Inatomi T, Sotozono C, et al. Ocular surface reconstruction using stem cell and tissue engineering. Prog Retin Eye Res 2016;51:187-207. [Crossref] [PubMed]
- Liang L, Sheha H, Li J, et al. Limbal stem cell transplantation: new progresses and challenges. Eye (Lond) 2009;23:1946-53. [Crossref] [PubMed]
- Kwitko S, Marinho D, Barcaro S, et al. Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology 1995;102:1020-5. [Crossref] [PubMed]
- Krakauer M, Welder JD, Pandya HK, et al. Adverse effects of systemic immunosuppression in keratolimbal allograft. J Ophthalmol 2012;2012:576712
- Biber JM, Skeens HM, Neff KD, et al. The cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea 2011;30:765-71. [Crossref] [PubMed]
- Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology 2002;109:1278-84. [Crossref] [PubMed]
- Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea 2011;30:1187-94. [Crossref] [PubMed]
- Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920-6. [Crossref] [PubMed]
- Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997;349:990-3. [Crossref] [PubMed]
- Kolli S, Ahmad S, Lako M, et al. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells 2010;28:597-610. [PubMed]
- Mariappan I, Maddileti S, Savy S, et al. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc 2010;5:1470-9. [Crossref] [PubMed]
- Ramirez BE, Sanchez A, Herreras JM, et al. Stem Cell Therapy for Corneal Epithelium Regeneration following Good Manufacturing and Clinical Procedures. Biomed Res Int 2015;2015:408495
- Cauchi PA, Ang GS, Azuara-Blanco A, et al. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol 2008;146:251-9. [Crossref] [PubMed]
- Baylis O, Figueiredo F, Henein C, et al. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem 2011;112:993-1002. [Crossref] [PubMed]
- Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea 2015;34:592-600. [Crossref] [PubMed]
- Sangwan VS, Basu S, MacNeil S, et al. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol 2012;96:931-4. [Crossref] [PubMed]
- Nakamura T, Endo K, Cooper LJ, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci 2003;44:106-16. [Crossref] [PubMed]
- Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 2004;351:1187-96. [Crossref] [PubMed]
- Alio del Barrio JL, Chiesa M, Garagorri N, et al. Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res 2015;132:91-100. [Crossref] [PubMed]
- Sanchez-Abarca LI, Hernandez-Galilea E, Lorenzo R, et al. Human Bone Marrow Stromal Cells Differentiate Into Corneal Tissue and Prevent Ocular Graft-Versus-Host Disease in Mice. Cell Transplant 2015;24:2423-33. [Crossref] [PubMed]
- Meyer-Blazejewska EA, Call MK, Yamanaka O, et al. From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 2011;29:57-66. [Crossref] [PubMed]
- Reza HM, Ng BY, Gimeno FL, et al. Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev 2011;7:935-47. [Crossref] [PubMed]
- Monteiro BG, Serafim RC, Melo GB, et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif 2009;42:587-94. [Crossref] [PubMed]
- Ouyang H, Xue Y, Lin Y, et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 2014;511:358-61. [Crossref] [PubMed]
- Hayashi R, Ishikawa Y, Sasamoto Y, et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016;531:376-80. [Crossref] [PubMed]
Cite this article as: Wang L, Wang B, Ouyang H. Limbal epithelial stem cells in corneal surface reconstruction. Ann Eye Sci 2018;3:3.


