
Treatment for diabetic macular oedema: looking further into the evidence
Introduction
The number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014 (1). Diabetic macular oedema (DMO) is a major sight-threatening complication of diabetes. Based on the estimated prevalence of DMO (~7% in diabetic patients) (2) there are at least 29.5 million people suffering from DMO worldwide. The prevalence of DMO increases with the duration of diabetes; it has been estimated that ~20% of diabetics will have DMO after 20 years of disease (2). The risk of developing DMO increases with poor glycemic control and with increased blood pressure and serum cholesterol levels (2). People diagnosed at age 30 or older with diabetes and who develop clinically significant macular oedema (CSMO), as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) criteria (see below), seem to have increased ischemic heart disease mortality (3).
Definition of DMO
Several definitions for DMO have been used over the years and should be taken into consideration when interpreting the results of published landmark randomised clinical trials (RCTs) on treatments for DMO.
Thus, DMO has been classified as CSMO/non-CSMO, focal/diffuse, ischaemic/non-ischaemic, centre/non-centre involving DMO, tractional/non-tractional, or mixed. Until relatively recently, CSMO/non-CSMO was the most widely definition used. CSMO was considered when thickening at/or within 500 microns from the centre of the fovea or hard exudation at/or within 500 microns from the centre of the fovea with adjacent retinal thickening or thickening of 1 disc area or more if within 1 disc diameter from the centre of the fovea, was present (4).
Based on findings on fundus fluorescein angiography (FFA) and depending on the extension of the area of leakage at the macula DMO can be classified as focal, when focal leakage from microaneurisms is detected, or diffuse, when diffuse leakage from diffusely dilated capillaries and/or from retinal pigment epithelium (RPE) throughout the macula is observed (5). There is no clear consensus with regards to the differentiation between ischaemic and non-ischaemic DMO. Some refer to ischaemic DMO whenever on FFA there is disruption of the perifoveal capillaries; others would refer to ischaemic DMO whenever areas of ischaemia are detected anywhere at the macula.
With the advent of optical coherence tomography (OCT) DMO was classified as centre or non-centre involving based on the presence or absence, respectively, of fluid at the centre of the fovea. Using also OCT, DMO was defined as tractional or non-tractional based on the presence/absence of a tractional component (either traction from an incompletely detached posterior hyaloid or from epiretinal membranes).
Given the different types of DMO and the different mechanisms for fluid accumulation, it would be extremely unlikely that a given treatment would be appropriate for all patients; a more personalised therapeutic approach would seem more appropriate for the treatment of patients with DMO.
Natural history
Although most cases of DMO will progress and fluid accumulation will worsen over time with subsequent visual loss, spontaneous resolution of DMO can occur (Figure 1). It has been suggested that spontaneous resolution of DMO may be observed in ~33–35% of patients (6). On this regard, it is interesting to note that in the sham arm of the RISE and RIDE RCTs, only 74% and 70% of patients, respectively, received macular laser suggesting that that ~30% of patients the DMO may have improved over the follow-up period requiring no treatment.
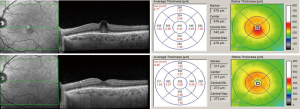
As it is not clear on whom DMO may resolve spontaneously, it is not possible to fully determine which patients could potentially be observed rather than treated.
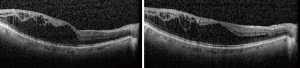
Unlike in the ETDRS study, recently conducted RCTs evaluating new treatments for DMO (see below) included only patients with some degree of visual loss (≤20/32 or ≤20/40) (7-10). However, not all patients with DMO will present with reduced vision. DMO, even when marked and centre-involving, may not cause immediate subjective or objective sight loss (Figure 2).

Furthermore, it is not clear when visual loss would ensue in a particular patient if DMO were to be left untreated and, importantly, when would sight loss become irreversible (Figure 3).
Current treatments
Prevention
Ideally, preventing the development of DMO, rather than treating it once established, should be the best option to avoid sight loss. Given that only a small proportion of all patients with diabetes will develop DMO, identifying the people at higher risk for developing this complication would be essential to increase the likelihood for potential preventive treatments to be cost-effective. As stated above, people with poor glycemic control, hypertension and high serum cholesterol are at higher risk of developing DMO; controlling these systemic risk factors should be sought, although may be difficult in some patients. Care should be taken, however, to avoid rapid changes (increase or decrease) in HbA1c and blood pressure as these may precipitate the development of DMO. Thus, at least in people with type 1 diabetes the risk of development of DMO requiring treatment was found to be reduced when changes in levels of HbA1c, systolic and diastolic blood pressure were kept within ±0.5%, 10 and 5 mmHg, respectively, during a 6-month period (11). Indeed, it has been recommended that six-month changes in HbA1c should be kept below 2 percentage points to minimise progression to CSMO (11-12).
Scarce data is available with regards to other characteristics that may make patients more prone to develop DMO; research studies attempting to elucidate risk biomarkers are, thus, very much needed.
Fenofibrate is an inexpensive drug to treat hypertriglyceridaemia. Two large RCTs, the fenofibrate intervention and event lowering in diabetes (FIELD) (13) and the Action to Control Cardiovascular Risk of Diabetes (ACCORD) (14) demonstrated the benefit of once-daily orally administered fenofibrate on the treatment of people with type 2 diabetes and diabetic retinopathy, including reducing the progression of the disease and the need for laser treatment for DMO and proliferative diabetic retinopathy (PDR). Fenofibrate has been licenced in Australia for the treatment of diabetic retinopathy. Given its low cost, safety profile and its beneficial effects not only on retinopathy but also on other diabetic complications (15-17) fenofibrate seems an excellent preventive measure for people with diabetes and diabetic retinopathy.
Treatment
As with regards to prevention, control of risk factors including HbA1c, blood pressure and lipids should be sought in patients with DMO. Communication with general practitioners and endocrinologists seems essential to achieve this.
Current treatment options for DMO include macular laser photocoagulation, intravitreal (anti-vascular endothelial growth factor) anti-VEGF therapies and intravitreal steroids. Herein, important considerations with regards to macular laser photocoagulation as well as anti-VEGF therapies for the treatment of DMO will be discussed. Although intraocular steroids are also a viable option on selected patients with DMO, as intravitreal steroids are not considered a first line therapy for the majority of patients, only few facts about this treatment modality will be presented.
Macular laser photocoagulation
The ETDRS = early treatment diabetic retinopathy study demonstrated that macular laser photocoagulation reduced the risk of losing ≥3 ETDRS lines by 50% at 3 years in people with diabetic retinopathy and DMO. However, a ≥15 letters improvement in vision was achieved only in <3% of patients (4). As a result, it has been repeatedly stated in the literature that laser can reduce the risk of moderate vision loss but cannot improve vision in DMO. It is essential however, to consider that in the ETDRS study, the definition used for the diagnosis of DMO was “CSMO”. Patients with CSMO may not have centre-involving DMO and, subsequently, may not have reduced vision. Furthermore, as discussed above, not all patients with centre-involving DMO will have either reduced vision. Indeed, in the ETDRS, the great majority of participants [1,903/2,243 eyes (85%)] had a baseline visual acuity of ≥20/40 at baseline (4). In contrast and for comparison, in recent landmark RCTs comparing laser with anti-VEGFs a baseline visual acuity of ≥20/40 was observed in ~20–50% of eyes only (7-10). As lower levels of vision prior to treatment appear to be associated with larger improvements in vision following treatment (8), the higher levels of vision observed in the ETDRS may have resulted in a reduced chance for visual acuity improvement. In fact, recent trials have shown that laser treatment can improve vision in people with DMO. Thus, in people with centre involving DMO, vision improved by ≥10 letters in 32% and 44% of patients at 2 and 3 years, respectively (18,19). The question is then, who are the laser responders?
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) appraised anti-VEGFs (ranibizumab and aflibercept) for the treatment of DMO (20,21). NICE found these treatments to be cost-effective compared with laser only in patients with centre involving DMO and central retinal thickness (CRT) ≥400 µ; in people with CRT <400 microns laser treated dominated and, as a result, laser treatment is recommended for the treatment of these latter patients in UK. CRT, thus, may assist in the selection of patients with higher chance to respond to laser treatment. On this regard, it is interest to note that in landmark trials comparing laser with anti-VEGFs the mean CRT at baseline was higher than 400 microns (405 µ in the DRCR.net protocol I, >460 µ RISE & RIDE, 412–426 µ in RESTORE, >479 µ VISTA & VIVID) (7,9,10,22). Laser photocoagulation may also be an appropriate option for patients with localised leakage on FFA. Other parameters that may make patients more suitable for laser treatment need to be elucidated. If DMO resolves following macular laser, recurrences are much less likely to occur than after anti-VEGF therapy and patients require to be seen much less often (follow-up intervals will be determined by the severity of the retinopathy) which is beneficial to patients, especially for those of working age who may find it difficult to take days off work to attend clinical appointments, and to health care providers, which are struggling to meet service demands.
In the EDTRS, FFA was advised prior to laser photocoagulation to guide this treatment. FFA can delineate areas of leakage or non-foveal ischaemia that should be treated with laser as well as determine whether perifoveal capillary drop-out is present, in which case laser treatment may not be appropriate. However, data does not exist with regards to whether or not outcomes following macular laser are improved by the use of FFA; research into this area is needed. Of note, in most recent landmark trials evaluating anti-VEGFs for DMO FFA was not required to guide macular laser photocoagulation.
In recent years, micropulse, subthreshold laser has gained popularity as a treatment strategy for exudative macular disorders. Preliminary data available suggests that this type of laser may be superior to standard laser for the treatment of patients with DMO (23,24). In micropulse subthreshold laser a series of short laser pulses are applied (“micropulse”), instead of a continuous wave emission as used in standard laser, with no obvious retinal burn. Each pulse is separated from the next one by a long off-time; this off-time has the purpose of allowing the tissue to cool down, avoiding an increase in temperature in the retina, as it occurs when conventional laser is used. In this manner, a sublethal effect on the RPE is achieved with no or minimal retinal damage. Small case series and randomised trials including relatively small number of patients have shown that subthreshold tissue-sparing micropulse laser may have comparable or higher efficacy than standard laser, even in the absence of a visible burn, with reduced side effects (23-27). This laser may be easier to deliver as it can be applied to the entire macular area, obviating the need to determine areas of leakage or retinal thickening. A large, adequately powered, pragmatic, multicentre, allocation concealed double-masked RCT is currently underway in the United Kingdom [diabetic macular oedema and diode subthreshold micropulse laser (DIAMONDS)] aimed at evaluating the clinical effectiveness and cost-effectiveness of diode subthreshold micropulse laser, when compared with standard threshold laser, for the treatment of patients with DMO) with a central retinal subfield thickness of (CST) of <400 microns (EudraCT number 2016-003804-29). DIAMONDS will provide robust evidence for the management of patients with these milder forms of DMO.
Anti-VEGF therapy
Several RCTs have consistently demonstrated the value of anti-VEGFs for the treatment of patients with centre involving DMO (CSMO was not used to define DMO in these studies) and its superiority over laser treatment.
It is likely that, on average, around 50–60% of patients on anti-VEGFs would be expected to improve ≥2 ETDRS lines (≥10 letter gain) following this therapy (7-9,22). However, the proportion of people and the degree of improvement in vision seems to be dependent on the existing level of vision when treatment is initiated (Tables 1 and 2). Thus, the recent protocol T of the DRCR.net demonstrated that a higher proportion of eyes with poorer levels of vision at presentation will experience visual acuity improvement (Table 1) (8). The degree of visual acuity improvement achieved seems to be also higher in eyes with poorer levels of vision (Table 2) (8).
Table 1
| Treatment | Initial VA (≥10 letter gain) (%) | |
|---|---|---|
| ≤20/50 | 20/32–20/40 | |
| Aflibercept-treated eyes | 77 | 50 |
| Bevacizumab-treated eyes | 60 | 45 |
| Ranibizumab-treated eyes | 69 | 50 |
VA, visual acuity.
Table 2
| Treatment | Initial VA | |
|---|---|---|
| ≤20/50 | 20/32–20/40 | |
| Aflibercept-treated eyes | 18.9±11.5 | 8.0±7.6 |
| Bevacizumab-treated eyes | 11.8±12.0 | 7.5±7.4 |
| Ranibizumab-treated eyes | 14.2±10.6 | 8.3±6.8 |
Anti-VEGF, anti-vascular endothelial growth factor; VA, visual acuity; SD, standard deviation
Many patients on anti-VEGFs, however, still require macular laser photocoagulation. Thus, in the DRCR.net protocol I, the proportion of eyes that required laser treatment in the ranibizumab + deferred laser arm increased over time, from 28% at 1 year to 42% at 2 years and 46% at 3 years (7,28,29). In the recently published DRCR.net. protocol T study, laser treatment was needed in 41, 52 and 64% of patients receiving aflibercept, ranibizumab and bevacizumab, respectively, at 2 years and after a median number of ~15 injections (30).
Over a third of patients in the ranibizumab arms had still central retinal thickening after 3 and 5 years of treatment (29,31). The 5-year data presented by the DRCR.net, Protocol I, however, should be interpreted with caution as only ~75% of patients randomised to the ranibizumab arms and evaluated a baseline remained in the study at 5 years (31). It is also of interest to note that, in the RISE and RIDE, where patients received anti-VEGF injections (ranibizumab) monthly for 2 years, leakage, as determined on FFA, was present in a very high proportion of patients (74-83%) at 2 years (10). This may suggest that although increased VEGF levels are a very important mechanism responsible for the pathogenesis of DMO, it may not be the sole cue, at least in a proportion of patients. Alternatively, the persistent leakage observed at 2 years despite monthly injections of anti-VEGF may relate to a short lived action of anti-VEGFs and subsequent rebound increased permeability 4 weeks after the last injection.
A high proportion of patients (38–48%) in the ranibizumab arms in the DRCR.net Protocol I RCT were still receiving injections in year 5, indicating that a prolonged treatment course is required in many patients.
Importantly, reduced diabetic retinopathy progression has been consistently detected following the use of anti-VEGF agents (7,10); this may possibly reduce rates of PDR in patients under this therapy.
Intraocular steroids
Triamcinolone, dexamethasone and flucinolone have been used for the treatment of patients with DMO.
The Protocol I of the DRCR.net showed that in pseudophakic eyes, treatment with triamcinolone + prompt laser achieved comparable visual benefit to Ranibizumab + laser (7). Importantly, triamcinolone + prompt laser seems to be more cost-effective than ranibizumab + laser (32). Triamcinolone, in its current formulation, however, if not licenced for intraocular use.
In UK dexamethasone and flucinolone have been appraised by NICE (33,34) for the treatment of DMO. Both have been recommended only for the treatment of pseudophakic eyes that do not respond to laser or anti-VEGFs.
Conclusions
Control of systemic risk factors, macular laser, anti-VEGFs and steroids can be used to treat patients with DMO. Treatment with fenofibrate should be considered to prevent the development of complications requiring treatment (DMO and PDR).
At least a third of patients will respond to laser treatment (very likely more if patients are selected for this treatment based on their characteristics e.g., those with localised leakage on FFA, those with <400 microns of CRT on OCT). Macular laser photocoagulation should be also perform in non-centre involving CSMO to prevent involvement of the centre and need for anti-VEGFs; the ETDRS study proved the value of laser under these circumstances. Laser should be also considered for patients with CSMO and normal or with minimal visual loss (>20/32) as data on anti-VEGFs is not available for this group but evidence from the ETDRS demonstrated a benefit of laser treatment.
In clinical practice it is expected that on average ~50–60% of patients will respond to anti-VEGFs with a ≥10 ETDRS letter improvement. Around 50% of patients will still require laser treatment and ~50% will still require injections 5 years on. The reduced progression of retinopathy observed in patients on anti-VEGF may lead to reduce rates of PDR in the future.
Licenced intraocular steroids (dexamethasone, flucinolone) may be considered as a second/third line therapy for people with DMO.
Ideally patients with DMO should be treated in a “personalised” manner, using the treatment preferred by the patient and the one that should provide the best chance to benefit her/him. Given that Health Services do not have infinite resources, not only the clinical effectiveness but also the cost-effectiveness of the treatments should be taken into consideration when selecting therapeutic strategies for patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Chi-Chao Chan and Mingguang He) for the series “Medical Retinal Diseases and Epidemiology” published in Annals of Eye Science. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2017.12.03). The series “Medical Retinal Diseases and Epidemiology” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global report on diabetes. World Health Organization 2016. Available online: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf
- Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556-64. [Crossref] [PubMed]
- Hirai FE, Knudtson MD, Klein BE, et al. Clinically significant macular edema and survival in type 1 and type 2 diabetes. Am J Ophthalmol 2008;145:700-6. [Crossref] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Arch Ophthalmol 1985;103:1796-806. [Crossref] [PubMed]
- Bresnick GH. Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology 1983;90:1301-17. [Crossref] [PubMed]
- Romero-Aroca P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care 2010;33:2484-5. [Crossref] [PubMed]
- Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372:1193-203. [Crossref] [PubMed]
- Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064-1077.e35. [Crossref] [PubMed]
- Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014;121:2247-54. [Crossref] [PubMed]
- Nguyen QD, Brown DM, Marcus DMRISE and RIDE Research Group, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789-801. [Crossref] [PubMed]
- Sander B, Larsen M, Andersen EW, et al. Impact of changes in metabolic control on progression to photocoagulation for clinically significant macular oedema: a 20 year study of type 1 diabetes. Diabetologia 2013;56:2359-66. [Crossref] [PubMed]
- Funatsu H, Yamashita H, Ohashi Y, et al. Effect of rapid glycemic control on progression of diabetic retinopathy. Jpn J Ophthalmol 1992;36:356-67. [PubMed]
- Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370:1687-97. [Crossref] [PubMed]
- Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014;121:2443-51. [Crossref] [PubMed]
- d'Emden MC, Jenkins AJ, Li L, et al. Favourable effects of fenofibrate on lipids and cardiovascular disease in women with type 2 diabetes: results from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2014;57:2296-303. [Crossref] [PubMed]
- Rajamani K, Colman PG, Li LP, et al. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet 2009;373:1780-8. [Crossref] [PubMed]
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849-61. [Crossref] [PubMed]
- Aiello LP, Edwards AR, Beck RW, et al. Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2010;117:946-53. [Crossref] [PubMed]
- Diabetic Retinopathy Clinical Research Network. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 2009;127:245-51. [Crossref] [PubMed]
- Ranibizumab for treating diabetic macular oedema. Available online: https://www.nice.org.uk/Guidance/ta274
- Aflibercept for treating diabetic macular oedema. Available online: https://www.nice.org.uk/Guidance/ta346
- Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE Study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118:615-25. [Crossref] [PubMed]
- Lavinsky D, Cardillo JA, Melo LA Jr, et al. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci 2011;52:4314-23. [Crossref] [PubMed]
- Vujosevic S, Bottega E, Casciano M, et al. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010;30:908-16. [Crossref] [PubMed]
- Kumar V, Ghosh B, Mehta DK, et al. Functional outcome of subthreshold versus threshold diode laser photocoagulation in diabetic macular oedema. Eye (Lond) 2010;24:1459-65. [Crossref] [PubMed]
- Figueira J, Khan J, Nunes S, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol 2009;93:1341-4. [Crossref] [PubMed]
- Laursen ML, Moeller F, Sander B, et al. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol 2004;88:1173-9. [Crossref] [PubMed]
- Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011;118:609-14. [Crossref] [PubMed]
- Diabetic Retinopathy Clinical Research Network. Intravitreal ranibizumab for diabetic macular edema with prompt vs deferred laser treatment: 3-year randomised trial results. Ophthalmology 2012;119:2312-8. [Crossref] [PubMed]
- Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, Bevacizumab, or ranibizumab for diabetic macular edema. Ophthalmology 2016;123:1351-9. [Crossref] [PubMed]
- Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomised trial results. Ophthalmology 2015;122:375-81. [Crossref] [PubMed]
- Dewan V, Lambert D, Edler J, et al. Cost-effectiveness analysis of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema: economic analysis of diabetic macular edema treatments. Ophthalmology 2012;119:1679-84. [Crossref] [PubMed]
- Dexamethasone intravitreal implant for treating diabetic macular oedema. Available online: https://www.nice.org.uk/guidance/ta349
- Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy. Available online: https://www.nice.org.uk/guidance/ta301
Cite this article as: Lois N. Treatment for diabetic macular oedema: looking further into the evidence. Ann Eye Sci 2018;3:2.


