
Clinical differences between young and older patients with optic neuritis
Introduction
Optic neuritis (ON) is an acute inflammatory optic neuropathy. It mostly occurs in individuals between the ages of 15 and 45 years, predominantly in females (1). Lots of previous studies focused on young patients or children (1-6). And most clinical knowledge about ON is based on the Optic Neuritis Treatment Trial (ONTT) (2), in which the investigators only enrolled young patients. Nevertheless, ON can occur at any age.
To the best of our knowledge, there is very little information about ON occurred in elderly patients (7,8). Choi et al. reported a case series of ON cases aged >50 years (7). They found that there was no clinical difference in ON between young and elderly patients. However, the sample size of their study was too small (only 8 cases) to reach a conclusion. Therefore, in the present study, we analyze more cases, and to determine whether clinical characteristics of ON in older patients are similar to young patients.
Methods
Clinical data were reviewed for the medical records of hospitalized patients diagnosed with ON who were admitted to the Department of Ophthalmology at the First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi from January 2003 to March 2014. Patients were included if they were: (I) Chinese; (II) aged between 18 and 70 years; (III) diagnosed with ON based on diagnostic criteria described elsewhere (9); (IV) had a duration between the symptom of ON and presentation at our clinic within 30 days. Patients were excluded if they had any evidence of the following conditions: (I) other optic neuropathies (e.g., hereditary, infiltrative, vascular, metabolic, toxic, traumatic, or radioactive optic neuropathy); (II) other ocular diseases (e.g., cataract, glaucoma and so on); and (III) systemic diseases (e.g., hypertension or diabetes mellitus).
Cases were identified into two groups: youth group (18–44 years) and older group (>44 years), then reviewed the data from each patient included age, sex, ocular symptoms, best corrected visual acuity (BCVA) at presentation, optic disc appearances, results of magnetic resonance imaging, treatment and the proportion of hypertension and hyperglycemia after corticosteroids therapy. Clinical parameters were compared between these groups.
We identified hypertension while systolic ≥140 mmHg or diastolic ≥90 mmHg (10). Fasting glucose ≥6.1 mmol/L or 2 hour glucose ≥7.8 mmol/L was identified hyperglycemia (11).
This study was approved by the institutional review board of the First Affiliated Hospital of Guangxi Medical University and followed the tenets of the Declaration of Helsinki. Written informed consent was not required because of the retrospective nature of this study.
Statistical analysis
Eyes without form vision, including no light perception, light perception, hand motion, and finger counting, were assigned decimal equivalents as previously described in our study (12). The best corrected decimal visual acuities were converted to the logarithm of minimal angle of resolution (LogMAR) scores for statistical analysis.
Statistical analyses were performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA). For comparison of clinical features in young and older patients, Pearson Chi-Square test, and Mann-Whitney U test were used as appropriate. All reported P values are two-tailed and statistical test with P<0.05 was considered significant.
Results
Demographic characteristics
During the study period, there were 184 ON cases (67 males, 117 females, 268 eyes) in total, including 133 young patients (51 males, 82 females) with median age of 30 years (range, 25–39 years) and 51 older patients (16 males, 35 females) with age of 54 years (range, 48–57 years). There was no statistically significant difference in gender between the youth group and older group (P=0.379) (Table 1).
Table 1
| Clinical characteristics | Young patient group (n=133) | Older patient group (n=51) | P values |
|---|---|---|---|
| Female | 82 (61.7%) | 35 (68.6%) | 0.379† |
| Age | 30 [25–39] | 54 [48–57] | NA |
| Unilateral involvement | 72 (54.1%) | 26 (51.0%) | 0.701† |
| Eye pain | 71 (53.4%) | 28 (54.9%) | 0.853† |
| Clinical evidence of MS | 9 (6.8%) | 4 (7.8%) | 0.799† |
| Optic disc swelling | 76 (57.1%) | 19 (37.3%) | 0.016† |
| Brain plaques | 10 (7.5%) | 11 (21.6%) | 0.007† |
| LogMAR scores|| | 2.05 (1.17–2.60) | 1.83 (0.88–2.90) | 0.255¶ |
| IVMP pulse therapy | 114 (85.7%) | 39 (76.5%) | 0.134† |
| Side effects†† | 7 (5.3%) | 15 (29.4%) | <0.001† |
*, data are median (quartile) or number (%); †, Pearson Chi-Square test; ||, LogMAR scores were converted from best corrected decimal visual acuities at presentation; ¶, Mann-Whitney U test; ††, only refer to hypertension or hyperglycemia. NA, not applicable; MS, multiple sclerosis; IVMP, intravenous methylprednisolone.
Clinical features
Among 184 patients with ON as shown in Table 1, 72 (54.1%) cases presented as unilateral ON in young group, while this proportion in older group was 26 (51.0%). Young patients had significantly more percentage of optic disc swelling than older patients (57.1% vs. 37.3%, P=0.016), while older patients had significantly more percentage of brain plaques than young patients (21.6% vs. 7.5%, P=0.007). All other parameters such as eye pain or clinical evidence of multiple sclerosis indicated no statistically significant difference between the two groups. We performed visual function measurements such as BCVA at presentation, BCVA in the older group appeared to be better than that in the youth group, but P values did not differ.
Treatment/hypertension or hyperglycemia caused by corticosteroids
In our study, 114 (85.7%) young patients and 39 (76.5%) older patients received 500–1,000 mg/d of intravenous methylprednisolone pulse therapy for 3–7 days, followed by oral prednisone tablets 1 mg/kg/d and gradually reduced the dosage. The rest of patients received other dosage of corticosteroids therapy. The distribution of corticosteroid use in two groups did not differ (P=0.134). We only focused on the proportion of hypertension and hyperglycemia, the side effects after corticosteroids treatment. The proportion of patients suffered from hypertension or hyperglycemia was nearly 6 times higher in the older group than in the young group (29.4% vs. 5.3%, P<0.001) (Table 1).
Discussion
ON is considered predominantly affects female patients (1), and 77.2% ONTT patients were female (2). Similarly, more female patients had ON compared with male counterparts in the youth and older group in the present study. This indicates that ON may be more likely to affect female cases than male in various ages, although no statistically significant difference was found in gender in our study.
We described the clinical features of young and older patients with ON, and this is the largest study involving ON patients of >44 years old. Choi et al. found that ON in elderly patients is uncommon, because only eight cases were diagnosed with ON in their study period (9 years) (7). However, we collected much more elderly ON cases (51 patients) in our study than Choi’ s report. The reason is probably that our hospital is the most famous clinical center in Guangxi province in south China, and much more patients visit it.
Why the incidence of optic disc swelling in older patients was lower than young patients in this study? The reason is unclear. As we know, optic disc swelling is the result of an inflammatory process (13). We speculate that hypoimmunity in elderly patients may weaken the inflammatory response. This might be the reason that the proportion of disc swelling in the older group is lower than the young group in the current study. Although we have reviewed a lot of literature (14-17), no study has been found about the relationship between anti-aquaporin-4 autoantibody and optic disc edema. However, in our clinical practice, ON patients without optic disc edema are associated with anti-aquaporin 4 antibody seropositive. Therefore, we speculated that the older patients are more likely having anti-aquaporin 4 antibody seropositive. This may be one of the reasons that the older patients have worse visual outcome (18).
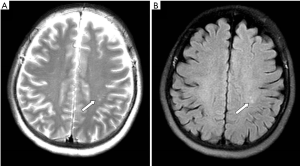
The present study revealed that older ON patients had more percentage of brain plaques, which has never been noticed before (Figure 1). Rizzo 3rd et al. found that elderly female patients had lower risk of developing multiple sclerosis than young female patients (19). According to our experiences, the reaction of white-matter for various harmful stimuli such as infection, poison, ischemia, hypoxia and nutritional deficiencies, can lead to brain plaques. Older individuals are more likely to suffer from such stimuli and have brain plaques, which is probably irrelevant to multiple sclerosis. Of course, the relationship between this phenomenon and multiple sclerosis in elderly ON patients needs further research.
One limitation of this retrospective study is that we did not undergo anti-aquaporin-4 autoantibody testing due to technical reasons. Therefore, we cannot determine which group are more likely to be associated with neuromyelitis optica, and we could not divide our patients according to the cause (20,21). To our knowledge, however, it is the largest case series regarding clinical characteristics of ON patients and comparing young and older cases.
In summary, there are some distinct differences between young ON patients (18–44 years) and older ON patients (>44 years). Compared with young patients, older patients have lower incidence of disc swelling, higher proportion of brain plaques and corticosteroids side effect. Since older patients are more susceptible to hypertension and hyperglycemia during corticosteroids therapy, we suggested physicians should pay attention to the side effects of corticosteroids in these patients in clinical practice.
Acknowledgments
Funding: This research was supported by the National Natural Science Foundation of China (No. 81560162) and Guangxi Natural Science Foundation (No. 2016GXNSFAA380301).
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aes.2017.10.01). They presented this manuscript as an oral presentation at 4th Chinese Neuro-Ophthalmological Society Conference in Beijing, China, in October, 2015.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the First Affiliated Hospital of Guangxi Medical University (No. 2017(KY-E-031)). Written informed consent was not required because of the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bee YS, Lin MC, Wang CC, et al. Optic neuritis: clinical analysis of 27 cases. Kaohsiung J Med Sci 2003;19:105-12. [Crossref] [PubMed]
- Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992;326:581-8. [Crossref] [PubMed]
- Wakakura M, Minei-Higa R, Oono S, et al. Baseline features of idiopathic optic neuritis as determined by a multicenter treatment trial in Japan. Optic Neuritis Treatment Trial Multicenter Cooperative Research Group (ONMRG). Jpn J Ophthalmol 1999;43:127-32. [Crossref] [PubMed]
- Zhou H, Wang W, Xu Q, et al. Clinical Features and Visual Outcomes of Optic Neuritis in Chinese Children. J Ophthalmol 2016;2016:9167361.
- Wilejto M, Shroff M, Buncic JR, et al. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology 2006;67:258-62. [Crossref] [PubMed]
- Roussat B, Gohier P, Doummar D, et al. Acute optic neuritis in children: clinical features and treatment. A study of 28 eyes in 20 children. J Fr Ophtalmol 2001;24:36-44. [PubMed]
- Choi J, Kim SJ, Chang JW, et al. Clinical characteristics of optic neuritis in Koreans greater than 50 years of age. Korean J Ophthalmol 2012;26:111-5. [Crossref] [PubMed]
- Qiu H, Wei S. Clinical analysis of the etiology of optic neuritis in patients at different ages in china. Eye Sci 2012;27:98-101. [PubMed]
- Du Y, Li JJ, Zhang YJ, et al. Risk factors for idiopathic optic neuritis recurrence. PLoS One 2014;9:e108580 [Crossref] [PubMed]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52. [Crossref] [PubMed]
- Lin YC, Yen MY, Hsu WM, et al. Low conversion rate to multiple sclerosis in idiopathic optic neuritis patients in Taiwan. Jpn J Ophthalmol 2006;50:170-5. [Crossref] [PubMed]
- Du Y, Ye H, Li K, et al. Vision-related quality of life tends to be more severely impaired in patients with dysthyroid optic neuropathy. Curr Eye Res 2014;39:532-6. [Crossref] [PubMed]
- Van Stavern GP. Optic disc edema. Semin Neurol 2007;27:233-43. [Crossref] [PubMed]
- Peng C, Wang W, Xu Q, et al. Thickness of macular inner retinal layers and peripapillary retinal nerve fibre layer in neuromyelitis optica spectrum optic neuritis and isolated optic neuritis with one episode. Acta Ophthalmol 2017;95:583-90. [Crossref] [PubMed]
- Zhou H, Xu Q, Zhao S, et al. Distinct clinical characteristics of atypical optic neuritis with seronegative aquaporin-4 antibody among Chinese patients. Br J Ophthalmol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Li H, Wang Y, Xu Q, et al. Features of anti-aquaporin 4 antibody-seropositive Chinese patients with neuromyelitis optica spectrum optic neuritis. J Neurol 2015;262:2293-304. [Crossref] [PubMed]
- Lai C, Tian G, Takahashi T, et al. Neuromyelitis optica antibodies in patients with severe optic neuritis in China. J Neuroophthalmol 2011;31:16-9. [Crossref] [PubMed]
- Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis. Experience of the Optic Neuritis Treatment Trial. Ophthalmology 1994;101:1771-8. [Crossref] [PubMed]
- Rizzo JF 3rd, Lessell S. Risk of developing multiple sclerosis after uncomplicated optic neuritis: a long-term prospective study. Neurology 1988;38:185-90. [Crossref] [PubMed]
- Matiello M, Lennon VA, Jacob A, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology 2008;70:2197-200. [Crossref] [PubMed]
- Jarius S, Frederikson J, Waters P, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci 2010;298:158-62. [Crossref] [PubMed]
Cite this article as: Luo W, Huang QS, He JF, Han M, Liu B, Du Y. Clinical differences between young and older patients with optic neuritis. Ann Eye Sci 2017;2:67.


